|
Case Report
A 39-year-old farmer
by profession reported dull pain (3–4/10 on the
Visual Analogue Scale) in the left lumbar area for
one year. An orthopaedic surgeon had previously
examined him, ruling out any conditions that would
have caused pain. Occasionally, he would use
over-the-counter pain relievers. There was no
other medical or surgical history of significance
in the past. On examination, there was no spinal
or lumbar deformity, and the renal angles were not
tender. The cough impulse over and inferior to the
left renal angle was, however, barely palpable.

|
| Figure
1: Ultrasound (USG ) image showing defect
in posterior fascia (red arrows) leading
to fatty left lumbar hernia (blue arrows).
|
An ultrasound study
of the left lumbar area revealed a defect in the
posterior fascia with herniation of
extraperitoneal fat (Figure 1), which increased
with the Valsalva manoeuvre. The neck of the
hernia measured approximately 13mm. There was no
herniation of bowel loops or other viscera. There
was no defect on the right side. A diagnosis of
Grynfelt-Lesshaft hernia was made, and due to a
lack of management experience with this rare
condition, the patient was attached to the
services of a tertiary healthcare facility, where
further imaging by MRI and open hernioplasty with
mesh implantation were successfully undertaken. At
the three-year follow-up, the patient was
satisfied and symptom-free.
|
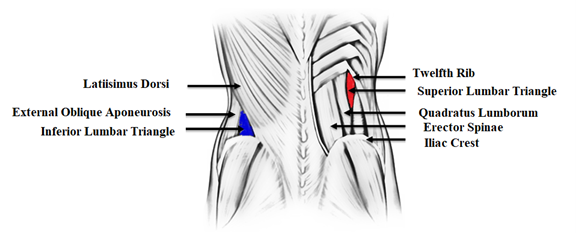
| Figure
2: Schematic diagram of anatomy of lumbar
area depicting superior lumbar triangle
(red colour) and inferior lumbar triangle
(blue colour) |
Discussion
Grynfelt-Lesshaft
hernia appears along the posterolateral abdominal
wall in the superior lumbar triangle (shown in red
in Figure 2), which is located posterior to the
lower pole of the left kidney. Posterior
(superficial) to this hernia is the latissimus
dorsi muscle, medial to it is the quadratus
lumborum muscle, and superior relation is formed
by the 12th rib [1]. In contrast, the inferior
lumbar triangle (shown in blue in Figure 2) is
bounded by the latissimus dorsi posteriorly, the
external oblique aponeurosis anteriorly, and the
iliac crest inferiorly [1].
It is a rare
condition, with only about 300 cases published in
the peer-reviewed literature, and most physicians
are not exposed to it during their training [2].
As a result, physicians frequently fail to
recognize this hernia or misdiagnose it as a
lipoma, which causes treatment delays and an
increase in morbidity [3]. Barbette had suggested
the possibility of lumbar hernias in 1672, and the
first case was reported by Garangeot in 1731. The
boundaries of the inferior and superior lumbar
triangles were delineated by Petit and Grynfeltt
in 1783 and 1866, respectively [4].
On the basis of
origin, lumbar hernias have been classified as
congenital (20%) or acquired (80%). The congenital
hernias are believed to form during embryologic
development, when the invasion of the somatopleure
by the aponeuroses of the layered abdominal
muscles results in the creation of potentially
weak areas If acquired, the hernias may be either
primary (55%) or else secondary (25%) [4]. The
most common cause of primarily acquired lumbar
hernias is increased intra-abdominal pressure,
with predisposing factors such as old age, chronic
lung disease, extremes of weight, muscular
atrophy, and professions that involve lumbar
constraints. Secondarily acquired lumbar hernias
are associated with trauma, prior surgical
incisions, and infection or abscess formation
[1,4].
The patient may be
asymptomatic or else present with discomfort,
dragging sensations, flank swelling, and, rarely,
gut obstruction. These hernias may grow to huge
dimensions and have an inherent tendency of
getting larger over time. Retroperitoneal fat,
kidney, colon, or, less frequently, small bowel,
omentum, ovary, spleen, or appendix, may be
contained in the hernia [4]. Impulses on coughing
may be visible or palpable over the hernia, and if
it contains bowel loops, auscultation may reveal
audible bowel sounds over it.
In the presented
case, the hernia was documented by
ultrasonography, though this modality may fail to
detect the hernia due to the presence of body fat
in this region and a lower index of suspicion [4].
CT-scan and MRI studies can lead to accurate
diagnosis by proper delineation of the anatomy and
identification of defects in the muscular and
fascial layers [4]. These imaging modalities can
also distinguish a hernia from other differential
diagnoses, such as hematoma, abscess, or
soft-tissue tumour [5].
Surgical repair of
these hernias should be undertaken as early as
possible to avoid incarceration and strangulation
[4]. Bowel incarceration is reported in 25% of
cases, though strangulation is rare due to the
wideness of the hernial neck [4]. Eliminating the
defect and building a sturdy, elastic abdominal
wall that can sustain the strain of regular
physical activity are the two main objectives of
hernia repair [4]. Due to the rarity of this
hernia, there is no clear consensus on the type of
repair, but the classic repair published in the
literature uses the open surgical approach, where
tension-free closure of the defect is performed
either directly or using prosthetic mesh [6]. A
transabdominal or extraperitoneal laparoscopic
approach are the alternatives. It is advantageous
for the mesh to be positioned extra-peritoneally
since no bony anchorage is required. The sound
physiological principle of diffusion of the entire
intra-abdominal pressure on each square inch of
the implanted mesh serves as the foundation for
laparoscopic transabdominal preperitoneal mesh
repair, which has also been documented in the
literature [7]. It is a tensionless repair that
offers the patient rapid recuperation and improved
cosmesis.
There is a proposal
by some experts to base the surgical approach on a
classification that takes into account six
characteristics of the hernia: size, location,
content, aetiology, muscular atrophy, and the
existence or otherwise of a recurrence [8]. If the
patient reports bowel obstruction and there is
doubt about the viability of the bowel, laparotomy
has been suggested as a surgical approach to allow
resection of the non-viable bowel and adequate
peritoneal lavage [9].
Acknowledgements
The patient's consent to the publication of this
case study and its accompanying images is
gratefully acknowledged by the author.
References
- Stamatiou D, Skandalakis JE, Skandalakis LJ,
Mirilas P. Lumbar hernia: surgical anatomy,
embryology, and technique of repair. Am
Surg. 2009 Mar; 75(3):202-7.
- Ploneda-Valencia CF, Cordero-Estrada E1,
Castañeda-González LG et al. Grynfelt-Lesshaft
hernia a case report and review of the
literature. Ann Med Surg (Lond). 2016
Apr;7:104-6.
- Ahmed ST, Ranjan R, Saha SB, Singh B. Lumbar
hernia: a diagnostic dilemma. BMJ Case Rep.
2014 Apr 15;2014:bcr2013202085. doi:
10.1136/bcr-2013-202085.
- Sharma P. Lumbar Hernia. Med J Armed
Forces India. 2009 Apr; 65(2):178-9.
- Meinke AK. Totally extraperitoneal
laparoendoscopic repair of lumbar hernia. Surg
Endosc. 2003;17:734–737.
- Shadhu K, Ramlagun D, Chen S, Liu L. Surgical
approach towards Grynfelt hernia: A single
center experience. Medicine (Baltimore). 2018
Aug; 97(33):e11928.
- Heniford BT, Iannitti DA, Gagner M.
Laparoscopic inferior and superior lumbar hernia
repair. Arch Surg. 1997;132:1141–1144.
- Moreno-Egea A, Baena E, Calle M,Martinez J,
Albasini J. Controversies in the current
management of lumbar hernias. Arch Surg. 2007;142(1):82–88.
- Stupalkowska W, Powell-Brett SF, Krijgsman B.
Grynfeltt-Lesshaft lumbar hernia: a rare cause
of bowel obstruction misdiagnosed as a lipoma. J
Surg Case Rep. 2017 Sep 7;2017(9):rjx173.
doi: 10.1093/jscr/rjx173.
|



















