|
Introduction
Coronavirus
disease 2019 (COVID-19) is caused by a novel
coronavirus, known as severe acute respiratory
syndrome coronavirus 2 (SARS-CoV2). It has
generated huge concern for the high mortality rate
and the lack of specific and effective treatment.
In India, total number of reported cases are
around 4 crores with mortality of 1.19% while
cases reported across Himachal Pradesh are 3 lakh
with death ratio of 1.35%. 1,2
The survivors of
COVID-19 continue to battle the symptoms of the
illness, long after they have been clinically
tested negative for the disease known as long
haulers. The most challenging part of this
pandemic is how to manage this COVID-19 sequelae
varying from mild fatigue and bodyaches to severe
forms requiring long term oxygen therapy and lung
transplantation due to lung fibrosis, significant
cardiac abnormalities and stroke leading to
impairment in quality of health and life. The most
critically ill patients in the context of
SARS-CoV-2 infection, develop acute respiratory
distress syndrome (ARDS). 3
Radiologically, most patients present with
bilateral ground glass opacities with or without
consolidation, with preference of lower lobes.
Pulmonary fibrosis can be idiopathic and
considered as age related fibroproliferative
disease but chronic inflammation may also be
involved in the pathogenesis of lung fibrosis. 4
Pulmonary fibrosis
is also a known sequela of severe and/or
persistent damage to lung. 5 Fibrosis
could be viewed as a consequence of a disordered
wound healing process and may be directly related
to the severity of an inciting event. 6
Various mechanisms of lung injury in COVID-19 have
been described, with both viral and immune
mediated mechanisms being implicated. 7
Lung fibrosis is
considered to be due to the abnormal healing of
the injured lung parenchyma. In COVID-19 patients,
possible sources of injury include cytokine storm
due to improper inflammatory response, bacterial
co-infections, and thromboembolic events causing
microvascular damage and endothelial dysfunction.
The renin-angiotensin system is also believed to
be involved due to the high affinity of SARS-CoV-2
viral spike protein to the angiotensin-converting
enzyme-2 (ACE2) receptor. 8
Since there are
large number of COVID-19 positive patients with
pulmonary involvement on CT scans in the setting,
the outcome of study about pulmonary fibrosis,
inflammatory markers and relation of dose and
duration of steroids could possibly help in
prognosticating COVID-19 positive patients and
help in risk differentiation of patients.
Aims & Objectives
Primary
Objective
To evaluate the
pulmonary fibrosis cases secondary to Covid-19
pneumonia and it’s relation with inflammatory
markers in patients presenting at Indira Gandhi
Medical College, Shimla
Secondary
Objectives
To describe the
relation of inflammatory markers with pulmonary
fibrosis
To describe the
relation of dose and duration of steroids with
pulmonary fibrosis
Materials and Methods
This was a
Prospective Cohort study and included RT-PCR
confirmed COVID-19 pneumonia patients reporting to
department of medicine, IGMC Shimla (H.P.)
Inclusion
Criteria
Patients (>18
years old) with COVID-19 pneumonia confirmed by
RT-PCR who were discharged from hospital with any
CTSS score (Total score= 25) at presentation were
included. The radiological and biochemical
characteristics of all patients were collected and
analyzed. Imaging features and distributions were
analyzed across different time points. Eligible
patients were followed up at three months and six
months after hospital discharge. Informed written
consent was obtained from every participant. In
the current study we analyzed all participants who
had attended the three month and six month follow
up visit.
Exclusion
Criteria
Patients who did
not give consent to the trial and had underlying
ILD, COPD and fibrosis were excluded.
Study
Period: One Year- 1 st
August 2021 – 31 st July 2022
Data
Collection: Participants who completed the
follow up and imaging up to six months were
included in the study. Demographic characteristics
like age, gender, preexisting health condition
(Hypertension, DM, CKD, CLD, COPD, Malignancy,
Immunosuppression) and smoking history were
documented.
Clinical
characteristics including main symptoms and signs
at admission were recorded. Laboratory indices
including TLC count, neutrophil lymphocyte ratio,
c reactive protein, ferritin, d-dimer and lactate
dehydrogenase were collected at admission and at
three and six months respectively. HRCT chest at
admission and follow up of three and six months
respectively, was done. Important management and
therapeutic data including dose and duration of
steroid therapy received during hospital stay was
recorded. Data regarding use of antifibrotic drugs
was also collected.
Statistical
Analysis: Data collected was entered into the
excel sheet for further processing and statistical
analysis. Continuous variables were expressed as
mean values and standard deviations while
categorical variables were presented as
proportions, percentages and 95 % confidence
interval. The patients were stratified into two
groups according to their CT- SS: Those with CT-SS
of 1-17 were considered mild whereas those with
CT-SS of 18-25 were considered severe. To find out
association between different variables
appropriate parametric and non-parametric test of
significance was applied depending on type and
normality of data. For association p value less
than 0.05 were considered as statistically
significant. It was done using Epi info version 7.
Results
Initially we
recruited 65 patients (>18 years old) with
COVID-19 Pneumonia confirmed by RT-PCR with any
CTSS on HRCT chest at presentation. Out of 65
patients, 15 patients were lost to follow up at
three months, 5 patients died, and 5 patients were
lost to follow up at six months. Eventually, the
data of 40 patients of COVID-19 Pneumonia with
follow up CT chest at 6 months was recorded and
analyzed. None of the patients we studied received
antifibrotic agents. The radiological and
biochemical characteristics were collected, and
results are as follows.
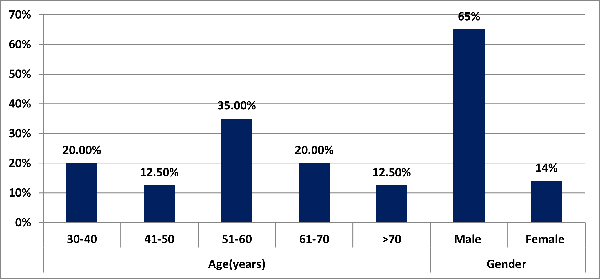
|
| Figure
1: Age and Distribution of study subjects.
|
Majority of patients
14(35.00%) belonged to age group 51-60 years
followed by 30-40 years [8(20.00%)] and 61-70
years [8(20.00%)]. Mean value of age(years) of
study subjects was 55.9 ± 13.7 with median
(25th-75th percentile) of 57.5(46.5-63.25).
Majority of patients 26(65.00%) were males
followed by females 14(35.00%)
| Table
1: Distribution of Clinical sign and
symptoms of study subjects |
|
Variables
|
Frequency
|
Percentage
|
|
Presenting symptoms
|
|
Fever
|
35
|
87.50%
|
|
Dry cough
|
37
|
92.50%
|
|
Shortness of breath
|
26
|
65.00%
|
|
Myalgia
|
7
|
17.50%
|
|
Loss of taste and smell
|
4
|
10.00%
|
|
Congestion or running nose
|
1
|
2.50%
|
|
Diarrhea
|
4
|
10.00%
|
|
Nausea or vomiting
|
0
|
0.00%
|
|
General physical examination
|
|
Pallor
|
2
|
5.00%
|
|
Icterus
|
1
|
2.50%
|
|
Cyanosis
|
10
|
25.00%
|
|
Clubbing
|
0
|
0.00%
|
|
Lymphadenopathy
|
0
|
0.00%
|
|
Edema
|
0
|
0.00%
|
|
JVP Raised
|
0
|
0.00%
|
|
Chest findings
|
|
Crepts
|
5
|
12.50%
|
|
Reduced breath sounds
|
24
|
60.00%
|
|
Vitals
|
Mean ± SD
|
Range
|
|
Systolic blood pressure(mmHg)
|
126.35 ± 14.26
|
108-170
|
|
Diastolic blood pressure(mmHg)
|
77.45 ± 8
|
60-98
|
|
Pulse rate (per minute)
|
86.52 ± 13.92
|
72-134
|
|
Respiratory rate (per minute)
|
29.1+5.9
|
20-36
|
|
SpO2(at room air)
|
80.72 ± 13.15
|
40-96
|
In majority
[37(92.50%)] of patients, presenting symptom was
dry cough followed by fever [35(87.50%)],
shortness of breath [26(65.00%)], myalgia
[7(17.50%)], loss of taste and smell [4(10.00%)],
diarrhea [4(10.00%)] and congestion or running
nose [1(2.50%)] In majority of In majority
[24(60.00%)] of patients, reduced breath sounds
was observed. Crepts were present in only 5 out of
40 patients (12.50%) patients, cyanosis
[10(25.00%)] was present followed by pallor
[2(5.00%)] and icterus [1(2.50%)] Mean value of
systolic blood pressure(mmHg), diastolic blood
pressure(mmHg), pulse rate (per minute),
respiratory rate (per minute) and SpO2 (at room
air) of study subjects was 126.35 ± 14.26, 77.45 ±
8, 86.52 ± 13.92, 29.1 ± 5.9 and 80.72 ± 13.15
respectively.
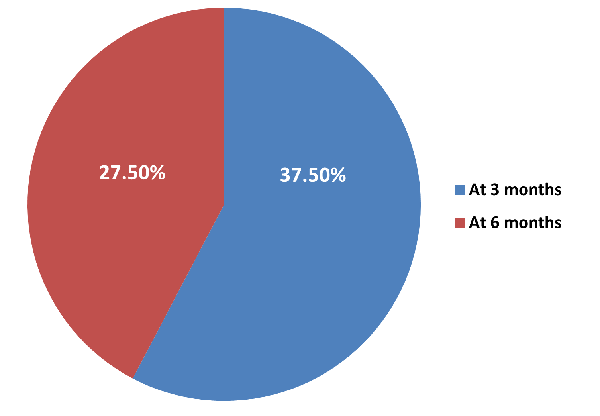
|
| Figure
2: Distribution of fibrosis of study
subjects. |
In 15(37.50%)
patients, fibrosis was present at 3 months and at
6 months; fibrosis was present in 11 (27.50%)
patients.
In our study,
32(80%) out of 40 patients had mild CTSS while
8(20%) patients had severe CTSS at presentation
| Table
2: Comparison of various biochemical and
radiological markers between mild and
severe lung involvement. |
|
Fibrosis
|
Mild (n=32)
|
Severe (n=8)
|
Total
|
P value
|
|
At 3 Months
|
|
No
|
25(78.13%)
|
0 (0%)
|
25(62.50%)
|
<.0001
|
|
Yes
|
7 (21.88%)
|
8 (100%)
|
15(37.50%)
|
|
At 6 Months
|
|
No
|
28 (87.5%)
|
1 (12.5%)
|
29(72.50%)
|
<.0001
|
|
Yes
|
4 (12.5%)
|
7 (87.5%)
|
11(27.50%)
|
Proportion of patients with fibrosis at 3 months
was significantly lower in mild as compared to
severe. (21.88% vs 100% respectively), which was
found to be statistically significant (p value
<0.0001) Proportion of patients with fibrosis
at 6 months was significantly lower in mild as
compared to severe.(12.5% vs 87.5% respectively)
which was found to be statistically significant (p
value <0.0001).
| Table
3: Association of Pulmonary Fibrosis with
Inflammatory Markers at 3 and 6 Months
|
|
|
At 3 months
|
At 6 months
|
|
Investigations
|
Fibrosis absent N=25 (N%)
|
Fibrosis present N=15 (N%)
|
P value
|
Fibrosis absent N=29 (N%)
|
Fibrosis present N=11 (N%)
|
P value
|
|
TLC
|
|
Normal
|
16(64)
|
9(36)
|
0.8
|
18(72)
|
7(28)
|
0.92
|
|
Deranged
|
9(60)
|
6(40)
|
11(73.3)
|
4(26.67)
|
|
QCRP
|
|
Normal
|
4(66.67)
|
2(33.3)
|
0.81
|
5(83.3)
|
1(16.7)
|
0.5
|
|
Deranged
|
21(61.76)
|
13(38.24)
|
24(70.59)
|
10(29.41)
|
|
NLR
|
|
Normal
|
5(100)
|
0
|
0.06
|
5(100)
|
0
|
0.1
|
|
Deranged
|
20(57.14)
|
15(42.86)
|
24(68.57)
|
11(31.43)
|
|
LDH
|
|
Normal
|
6(85.7)
|
1(14.29)
|
0.16
|
7(100)
|
0
|
0.07
|
|
Deranged
|
9(57.58)
|
14(42.42)
|
22(66.67)
|
11(33.3)
|
|
D DIMER
|
|
Normal
|
19(76)
|
6 (24)
|
0.02*
|
20(80)
|
5(20)
|
0.17
|
|
Deranged
|
6(40)
|
9(60)
|
9(60)
|
6(40)
|
|
FERRITN
|
|
Normal
|
9(90)
|
1(10)
|
0.03*
|
10(100)
|
0
|
0.02*
|
|
Deranged
|
16(53.33)
|
14(46.67)
|
19(63.33)
|
11(36.67)
|
|
Dexamethasone duration in days
(mean ±Standard deviation)
|
|
Dexamethasone duration
|
6.7(±3.94)
|
9.9(±1.79)
|
0.0047*
|
7(±3.8)
|
10.3(± 1.7)
|
0.009*
|
Fibrosis was
present in majority of patients at three months
with deranged TLC, QCRP, NLR, LDH and D-dimer and
ferritin, which was found to be statistically
significant (p value=0.02 and 0.03 respectively).
Fibrosis was present in majority of patients at
six months with deranged QCRP, NLR, LDH, D-dimer
and also ferritin, which was found to be
statistically significant (p value=0.02). On
comparing the presence of fibrosis at 3 and 6
months with dose and duration of steroids given
during the admission, we found that fibrosis was
absent in majority of patients (80%) when the dose
of dexamethasone was 6 mg per day as compared to a
higher dose (12 mg per day), where only 4%
patients did not develop fibrosis (p value
=<0.00001). Fibrosis at 3 and 6 months was
absent when steroids were given for a duration of
6.7 days and 7 days respectively when compared to
longer duration of steroids ( 9.9 days and 10.3
days respectively) (p value = 0.0047 and 0.009
respectively)
Discussion
HRCT played
important role in diagnosis and assessment of
disease severity. Among the various COVID-19
pulmonary sequelae, pulmonary fibrosis, which
develops due to abnormal healing of injured lung
parenchyma9, is one of the key concerns
as it decreases quality of life. Experiences with
SARS and MERS showed that follow up CT is advised
in patient recovering from COVID-19 to find out
which group of patients is more likely to develop
pulmonary fibrosis. Zou and colleagues showed that
30 and 90 day follow up of PCPF patients confirmed
that pulmonary fibrosis in some patients will
resolve over time; however in majority of
patients, it will not resolve10. Much
research has been done on pulmonary fibrosis.
However, outcome of fibrosis in post recovery
phase and its long-lasting effect on lung
parenchyma is still largely unanswered.
This study was
conducted in the Medicine department of IGMC
Shimla, Himachal Pradesh to describe the outcome
of pulmonary involvement in COVID-19 patients,
with baseline HRCT chest done, who were discharged
after treatment and followed up at 3 and 6 months
for fresh or worsening symptoms and radiological
changes in HRCT Chest. Then we compared the dose
and duration of steroids given at presentation and
the inflammatory markers with the radiological
changes at three and six months. In our study,
based on CT severity scoring at presentation we
divided patients into mild (CTSS- 17 or less) and
severe group (CTSS-18 or more) and studied the
prevalence of fibrosis at three and sixth months
in relation to CT severity score at presentation
along with inflammatory markers. We also studied
the relation of dose and duration of steroids at
presentation with pulmonary fibrosis.
Out of the 40 cases
we included in our study who completed six months
follow up, 26(65%) were males and 14(35%) were
females. The male to female ratio was 1.85. This
is comparable to a study carried out by Manuel
Taboada et al 11 in Spain in which 62%
patients were males and rest 38% patients were
females, the male to female ratio being 1.63. In
the study carried out by Xiaoyu Han et al12.
in Wuhan, People’s Republic of China, the male to
female ratio was 2.33However, in a study done in
Abu Dhabi, UAE by Ghufran et al 13 in
2021, the males were 85.3%, the females were 14.7%
were females, the male to female ratio being 5.66.
The mean age of
study population in our study was 55.9+13.7
years. This was similar to study done by Xiaoyu
Han et al 12 where mean age of the
patients was 54+12 years and it was found
that age more than 50 years was an independent
predictor for fibrotic like changes in the lung at
6 months. Another study by Chen et al 14
where mean age of the patients was 41.9+13.3
years.
In our study,
32(80%) out of 40 patients had mild CTSS while
8(20%) patients had severe CTSS at presentation
(Mean+SD=10.85+5.89). 15(37.5%) out
of 40 participants who recovered from COVID-19
pneumonia developed fibrotic like changes in the
lung at three months out of which 7(21.8%)
patients were in mild group whereas 8(100%)
patients were in severe group while at six months,
11(27.5%) patients developed fibrosis; in this
group 7 patients (87.5%) were in severe group
while 4 patients (12.5%) belonged to mild group.
Proportion of patients with fibrosis at 6 months
and 3 months was significantly lower in mild as
compared to severe group (p value<0.0001).
Similar prospective
study was conducted by Xiaoyu Han et al 12
where pulmonary sequelae of COVID-19 patients was
assessed and follow up HRCT chest were done at 17+11
days and 175+20 days in which CT severity
score of patients was calculated. 40 of the 114
participants (35%) develop fibrotic like changes;
in this group, most of the fibrotic like changes
(22 of 40- 55%) manifested at 6 month follow up
whereas 74(65%) showed either complete radiologic
resolution or residual ground glass opacification.
Similar to our study higher CT score (>18)
on initial CT scan was independent predictor of
development of fibrotic like changes in the lung
after 6 months follow up. The laboratory results
also showed higher d dimer and c reactive protein
levels in patients with fibrotic like changes
while in our study, fibrosis was present in
majority of patients at three months with deranged
TLC, QCRP, NLR, LDH (40%, 38.24%, 42.86% &
42.42% respectively) and also D-dimer and ferritin
(60% & 46.67% respectively), which was found
to be statistically significant(p value=0.02 and
0.03 respectively) and fibrosis was also present
in majority of patients at six months with
deranged QCRP, NLR, LDH, D-dimer (29.9%, 31.43%,
33.3% & 40% respectively) and also ferritin
(36.67%), which was found to be statistically
significant (p value=0.02) The difference in
outcome of our study and the above-mentioned study
was primarily due to small sample size. Also, the
extent of fibrosis was not quantified in both the
studies.
Another study was
conducted by Rabab Yasin et al 15 in
Egypt including 210 patients to predict lung
fibrosis in Post COVID-19 patients. At least one
follow up chest CT was done at 20-65 days after
discharge and it showed fibrosis in 48.1% patients
while 51.9% had no residual fibrosis with majority
of patients with fibrosis in older age group,
comparable to our study. The patients with
fibrosis also had higher rate of ICU admissions,
the factor which was not included in our study.
They also reported higher level of inflammatory
markers- c-reactive protein, d-dimer, ferritin in
patients with fibrosis similar to our study
suggesting that deranged inflammatory markers are
more associated with pulmonary fibrosis. Patients
(83.8%) were given pulse steroid therapy in
contrast to our study where dose and duration of
steroid therapy was also evaluated in relation to
fibrosis.
We described
outcome of COVID-19 based on presence or absence
of fibrosis at 6 months and found out that
fibrosis was absent in majority of patients when
dose of dexamethasone given was 6 mg (80%)
compared to 12 mg (4%) (p value <0.00001).
Also, we found that the mean duration of steroids
6.7+3.94 days and 7+3.8 days was
associated with absent fibrosis at three and six
months respectively, which was statistically
significant (p value=0.0047 and 0.009
respectively). Contrary to our study, Manuel
Taboada et al 11 conducted a study
where 200 patients were assigned in 1:1 ratio to
receive low dose (6 mg) once daily for 10 days and
high dose (20mg) once daily for five days followed
by 10 mg for additional 5 days. They found that
high dose of dexamethasone reduced clinical
worsening within 11 days after randomization,
compared with low dose. Different sample size and
duration of steroids in the above-mentioned
studies was the main difference in the outcome of
these two studies.
Limitations
Firstly, sample
size was very small and follow up was done for
only 6 months. Patients with fibrotic like changes
require longer follow up to determine whether
these changes are permanent or reversible. Only
semi quantitative scores in the form of CT
severity score was used which was shown to be
correlated with the degree of pulmonary fibrosis.
The extent of fibrosis was not quantified. Lack of
histologic correlation is also a limitation.
Therefore, further studies are needed to find
whether fibrotic like changes on CT scans
represent true pathologic fibrosis.
Therefore,
identifying the predictive factors for pulmonary
fibrosis such as higher CTSS in initial HRCT
chest, raised inflammatory markers at presentation
and high dose of steroid (12mg dexamethasone) for
a longer duration in clinical practice can help in
preventing the development and progression of lung
fibrosis.
Conclusion
In conclusion, most
of the patients with mild lung involvement
(CTSS-17 or less) at presentation, fibrosis was
significantly lower at 3 months and 6 months of
follow up in comparison to patients with severe
lung involvement (CTSS>17). At 3 months
fibrosis was present in majority of patients with
deranged TLC, QCRP, NLR, LDH and also D-dimer and
ferritin which was found to be statistically
significant. At 6 months fibrosis was present in
majority of patients with deranged QCRP, NLR, LDH,
D-dimer along with ferritin which was significant.
Our study also suggested that steroids for an
average duration of 10 days at presentation was
significantly associated with improvement in
fibrosis.
References
- Zumla A, Hui DS, Azhar EI, Memish ZZ, Maeurer
M. Reducing mortality from 2019-nCoV: host
directed therapies should be an option. The
Lancet. 2020;395: e35-e36.
- COVID 19 state wise status. Available at: https://www.mohfw.gov.in/
[last assessed on 19th December
2022].
- Guan WJ, Ni ZY, HuY, Liang WH et al. China
medical treatment expert group for Covid-19:
Clinical characteristics of coronavirus disease
2019 in china. N Engl J Med. 2020;382:
1708-1720.
- Wang D, Hu B, Hu C, Zhu F, Liu X et al:
Clinical characteristics of 138 hospitalized
patients with 2019 novel coronavirus infected
pneumonia in Wuhan, China, JAMA.
323(1061) 2020.
- Wilson MS, Wynn TA. Pulmonary fibrosis:
pathogenesis, etiology and regulation. Mucosal
Immunology. 2009;2(2):103–121.
- Taskar V, Coultas D. Exposures and idiopathic
lung disease. Seminars in Respiratory and
Critical Care Medicine. 2008;29(6):
670–679.
- Liu J, Zheng X, Tong Q, Li W, Wang B, Sutter
K, Trilling M, Lu M, Dittmer U, Yang D.
Overlapping and discrete aspects of the
pathology and pathogenesis of the emerging human
pathogenic coronaviruses SARS-CoV, MERS-CoV, and
2019-nCoV. J Med Virol. 2020
May;92(5):491-494. doi: 10.1002/jmv.25709.
- Hama Amin BJ, Kakamad FH, Ahmed GS et al. Post
COVID-19 pulmonary fibrosis; a meta-analysis
study. Ann Med Surg (Lond). 2022
May;77:103590. doi: 10.1016/j.amsu.2022.103590.
- Fernandez IE, Eickelberg O. New cellular and
molecular mechanisms of lung injury and fibrosis
in idiopathic pulmonary fibrosis. Lancet.
2012 Aug 18;380(9842):680-8.
- Zou JN, Sun L, Wang BR, Zou Y, Xu S, Ding YJ
et al. The characteristics and evolution of
pulmonary fibrosis in COVID-19 patients as
assessed by AI- assisted chest HRCT. PLoS
One. 2021;16(3):1–12.
- Taboada M, Rodríguez N, Varela PM, Rodríguez
MT et al. Effect of high versus low dose of
dexamethasone on clinical worsening in patients
hospitalized with moderate or severe COVID-19
pneumonia: an open-label, randomized clinical
trial. Eur Respir J. 2022 Aug
4;60(2):2102518.
- Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M,
Li Y, Cao Y, Gu J, Wu H, Shi H. Six-month
Follow-up Chest CT Findings after Severe
COVID-19 Pneumonia. Radiology. 2021
Apr;299(1): E177-E186.
- Saeed GA, Gaba W, Shah A, Al Helali AA et al.
Correlation between Chest CT Severity Scores and
the Clinical Parameters of Adult Patients with
COVID-19 Pneumonia. Radiol Res Pract. 2021
Jan 6:6697677.
- Chen W, Zheng KI, Liu S et al. Plasma
CRP level is positively associated with the
severity of COVID-19. Ann Clin Microbiol
Antimicrob. 2020 May 2015; 19(1):18.
- Yasin R, Gomaa AAK, Ghazy T, Hassanein SA,
Ibrahem RAL, Khalifa MH. Predicting lung
fibrosis in post-COVID-19 patients after
discharge with follow-up chest CT findings. Egypt
J Radiol Nucl Med. 2021;52(1):118.
|



















