|
Introduction
Gestational
diabetes mellitus (GDM) is defined as glucose
intolerance of varying severity that occurs first
time, or is first detected during pregnancy [1].
Prevalence of GDM is 7% of all pregnancies in the
world [2]. GDM is one of the leading causes of
morbidity and mortality in both mother and
new-borns worldwide [3]. In India, its prevalence
is found to vary in urban and rural areas.
However, a recent study by Choudhary et al
reported a prevalence of 9% in India [4].GDM is
commonly associated with infections of the urinary
tract.
Asymptomatic
bacteriuria (ASB) refers to the presence of
bacteria in the urine without any symptoms of a
UTI, while pyuria is the presence of an elevated
number of white blood cells in the urine,
indicating potential inflammation or infection in
the urinary tract. Both conditions are important
to consider when evaluating urinary tract health
and may require further investigation and
management, depending on the individual's clinical
characteristics and risk factors.
Urine culture is
considered the gold standard for diagnosing ASB.
It involves incubating urine samples on culture
media to detect bacterial growth. The presence of
a significant colony count of a single
uropathogenic organism typically indicates ASB.
Urine culture is generally highly sensitive,
capable of detecting low bacterial loads, and is
considered the benchmark for assessing sensitivity
of other techniques. The specificity of urine
culture is also high as it can differentiate
between pathogenic and commensal bacteria.
However, urine
culture has some limitations. It is
time-consuming, usually requiring 24 to 48 hours
for results. Additionally, some fastidious
bacteria may not grow on standard culture media,
leading to false negatives. Moreover, it may not
be practical in certain settings, such as
point-of-care testing.
Heparin-binding
protein (HBP) is a biomarker associated with
inflammation and tissue damage. ELISA-based assays
can measure the concentration of HBP in urine
samples, which may indicate the presence of
inflammation in the urinary tract. Specificity for
UHBP has been reported to be higher than
sensitivity in some studies, suggesting that false
positives might be less frequent. One limitation
of UHBP measurement is that elevated levels can be
seen in other inflammatory conditions, not
specific to ASB. Therefore, the diagnostic
accuracy of UHBP needs to be carefully assessed.
Multiplex PCR is a
molecular technique that can amplify and detect
DNA sequences of multiple bacterial species
simultaneously. It is highly sensitive and can
detect even low bacterial loads.
Urinary tract
infections (UTI) are one of the most common
infections resulting in a significant healthcare
expenditure. Pregnant women because of the
physiological changes are highly susceptible to
UTI [5]. During pregnancy, the treatment of UTI is
recommended even if there no accompanying
symptoms, i.e., Asymptomatic bacteriuria (ASB).
It has been claimed
ASB is three to four times more common in women
with diabetes than in healthy women [6].
Prevalence of ASB is reported to be as high as 30%
in diabetic women [7]. ASB is considered
clinically significant and worth treating during
pregnancy because treatment will effectively
reduce the risk of pyelonephritis and preterm
delivery [8]. UTI are dependent on the accurate
identification of pathogens. Even though urine
culture is the gold standard test, it takes 24-48
hours to give results. Some organisms can be
fastidious and therefore difficult to grow in
culture. So it is need of the hour to look for an
alternative urinary biomarker which correlates
with the urine culture results. It is also
important to look for a technique which gives
accurate results in a short time interval.
Further, PCR results can be obtained in a day or
less, while culture can require 2 or more days
[9]. In the present study, we compared three
different methods such as urine culture, Urinary
Heparin Binding Protein (UHBP) assay by ELISA and
identification of pathogens by multiplex PCR for
the diagnosis of ASB/UTI in GDM. Gestational
diabetes, which is a combination of pregnancy and
diabetes are at the high risk of ASB as well as
UTI. Therefore, it is justifiable to detect a
biomarker or technique which can detect ASB/UTI in
a short span of time.
Objectives:
- To find the prevalence of
asymptomatic bacteriuria/pyuria in gestational
diabetes mellitus
- To find the association between asymptomatic
bacteriuria/pyuria and urinary heparin binding
protein (UHBP)
- To compare sensitivities and specificities of
the techniques (urine culture and UHBP by ELISA
and multiplex PCR)
Methods
Study
design and participants:
We included 50 GDM
patients diagnosed based on 75 gm oral GTT (OGTT)
as per modified DIPSI criteria who are willing to
participate in this observational study. Samples
were collected from OBG department (OP & IP)
from Justice K. S. Hegde Charitable Hospital,
Deralakatte, Mangalore and the study was conducted
in Microbiology and Molecular genetics laboratory
of Central Research Lab, K. S. Hegde Medical
Academy, Nitte (Deemed to be University),
Mangaluru, Karnataka, India.
Exclusion
criteria and Ethics: Women with multiple
pregnancy, known pre-gestational diabetes,
pregnancies complicated by major foetal
malformations or known major cardiac, renal or
hepatic disorders were excluded.
Institutional
Ethics Committee approval was obtained prior to
the study. Written Informed Consents were taken
from the patients
Laboratory
Investigations
Urine
culture
Clean catch
midstream urine samples were collected into a
sterile screw capped urine container by standard
method. The samples were labelled and 0.2 mg of
boric acid was added to prevent the bacterial
growth in urine samples. The samples were cultured
on cysteine-lactose electrolyte deficient agar and
blood agar using a sterile 4 mm platinum wired
calibrated loop for the isolation of
microorganisms. The plates were incubated for
overnight at 37 °C and the samples were considered
positive when an organism cultured is at a
concentration of 10 5 CFU/mL which was
estimated through multiplying the isolated
colonies by 1000. The isolates were identified up
to the species level by standard biochemical
tests.
Antibiotic
sensitivity testing:
Antibiotic
sensitivity testing was performed by the modified
disc diffusion method. Inoculums adjusted to 0.5
McFarland standard was swabbed on Mueller Hinton
agar plates for antibiotic sensitivity assay.
Eight groups of antimicrobials such as Amoxyclav,
Cephalexin, Nitrofurantoin, Erythromycin,
Sulfisoxazole, Fosfomycin, Imipenem and
Trimethoprim / Sulfamethoxazole were selected and
sensitivity pattern was tested.
Estimation
of UHBP by ELISA
Tubes were
centrifuged within 1 hour of sampling at 3000 rpm
for 10 minutes, and separate aliquots of the
supernatant were stored at -20°C until analysis
for UHBP. UHBP was estimated using ELISA.
Characterization
of Pathogen by Multiplex PCR
DNA Extraction
and Analysis
DNA was extracted
from urine samples using the MagMAX DNA
Multi-Sample Ultra Kit. One ml of urine sample was
added into 2.0ml collection tube. Centrifuged for
2 minutes at 13,000 rpm. The supernatant was
discarded completely. The pellet obtained was
resuspended using 200μl of Resuspended Solution
(1X PBS) (ML116) and mixed well. 20μl of RNase A
solution was added, mixed and incubated for 2
minutes at room temperature (15-25ºC). Cell lysis
was carried out by adding 20μl of Proteinase K
solution followed by 200μl of Lysis solution to
the re-suspended pellet. Mixed for 15 minutes
using a Vertex. Incubated for 10 minutes at 70ºC.
200μl of ethanol (96-100%) was added to the lysate
and mixed with help of vertex for 5 to 10 seconds.
Lysate obtained was transferred to the HiElute
Miniprep Spin Column. Centrifuged at 10,000 rpm
for 1 minute. The flow through liquid was
discarded and the column was placed in a same
2.0ml collection tube. About 500μl of diluted wash
solution was added to the column and centrifuged
at 10,000 rpm for 1 minute. The flow- through
liquid was discarded and the same collection tube
was re-used with the column. Again 500μl of
diluted wash solution was added to the column and
centrifuge at 13,000 for 3 minutes to dry the
column. The column was centrifuged for another 1
minute at same speed to dry the residual ethanol
(as required). 200μl of Elution buffer was added
directly onto the column, incubated for 1 minute
at room temperature (15-25ºC). Centrifuged at
10,000 rpm for 1 minute to elute the DNA. The
isolated DNA was transferred to a fresh 2ml
collection tube with a cap and stored -20ºC.
Multiplex PCR
HiMedia’s Hi-PCR
Sepsis Pathogen Semi-Q PCR Kit (Multiplex) was
used for the amplification of specific gene using
specific primers in a single tube reaction. The
PCR Master mix was prepared as indicated in the
Table1 . Components listed in the table 1
were added to centrifuge tube and was centrifuged
at 6000rpm for about 10 seconds. The tubes were
placed in MiniAmp Plus Thermal cycler and PCR
program was set as listed in the Table 2
. For analysis of the PCR data, 10μL of amplicon
were loaded on a 1.5% agarose gel (incorporated
EtBr) along with 1μL of 6X Gel loading buffer. 3μL
of 50bp DNA ladder was used. Organisms detected
and their band sizes are listed in the Table
3.
|
Table 1: Protocol for PCR
Master mix preparation
|
|
Components
|
Volume (μL) to be added for 1R (for a
50μL reaction)
|
|
Tube 1
|
Tube 2
|
|
2 X PCR TaqMixture
|
25 μL
|
25 μL
|
|
Sepsis Primer Mix 1
|
6 μL
|
_
|
|
Sepsis Primer Mix 2
|
_
|
6 μL
|
|
Template DNA/Positive Control/ Negative
control
|
5 μL
|
5 μL
|
|
Total volume
|
Up to 50 μL
|
Up to 50 μL
|
|
Table 2: PCR program
|
|
Initial denaturation
|
94ºC 3 minutes
|
|
denaturation
|
94ºC 1 minute
|
|
Annealing
|
58ºC 1 minute
|
|
Extension
|
72ºC 1 minute
|
|
Final Extension
|
72ºC 5 minutes
|
|
Table 3: Data
interpretation.
|
|
Organism
|
Band size
|
|
E. coli
|
879bp
|
|
S. aureus
|
450bp
|
|
K. pneumonia
|
324bp
|
|
P. aeruginosa
|
961bp
|
|
S. typhi
|
606bp
|
|
A. baumannii
|
490bp
|
Statistical
Analysis
The statistical
analysis was carried out with SPSS 21.0. and
GraphPad Prism 9. Receiver operating
characteristic (ROC) curves were constructed to
compare sensitivities, specificities, positive
predictive values and negative predictive values
of the three techniques.
Results
Total of 50
patients with GDM were enrolled during first year
with the mean age of 29.3±4.27years. Their mean
FBS value was 157 ± 17.2 mg/dl. Prevalence of ASB
among GDM patients on basis of urine culture is
listed in Table 5. Prevalence of ASB
among GDM patients on basis of Multiplex PCR is
demonstrated in Table 6. A significant
association was noted between detection of
organisms and multiplex PCR findings compared to
urine culture (p <0.0001) as indicated in
Table 7 .
|
Table 5:
Prevalence of asymptomatic bacteriuria
in GDM as per urine culture
|
|
Name of organism
|
N
|
%
|
|
Acinetobacter lowffi
|
1
|
2
|
|
Enteroco fecalis
|
1
|
2
|
|
Klebsiella
|
1
|
2
|
|
Contaminant
|
1
|
2
|
|
Mixed flora
|
17
|
34
|
|
No Growth (NG)
|
7
|
14
|
|
Non-significant bacteria (NSB)
|
22
|
44
|
|
Table 6:
Prevalence of asymptomatic bacteriuria
in GDM as per Multiplex PCR
|
|
Name of organism
|
N
|
%
|
|
Acinetobacter baumanii
|
1
|
2
|
|
E. coli
|
2
|
4
|
|
E. coli + Acinetobacter
|
1
|
2
|
|
E. coli + Klebsiella + Acinetobacter
baumani + Pseudomonas
|
1
|
2
|
|
E. coli + Klebsiella + Salmonella
|
1
|
2
|
|
E. coli + Klebsiella +
Salmonella + Pseudomonas
aeroginosa
|
1
|
2
|
|
E. coli + Staphylococcus
aureus
|
3
|
6
|
|
Klebsiella
|
3
|
6
|
|
No growth
|
14
|
28
|
|
Staphylococcus aureus
|
13
|
26
|
|
Staphylcoccus aureus + E.
coli
|
1
|
2
|
|
Staphylococcus aureus + Klebsiella
|
9
|
18
|
Median (25% - 75%)
value of UHBP levels in urine was noted to be 689
(625, - 863) pg/ml. A significant association was
observed between UHBP levels and multiplex PCR
findings with (p= 0.017) (Table 7). No association
was found between UHBP levels and urine culture
reports (p<0.99) (Table 8).
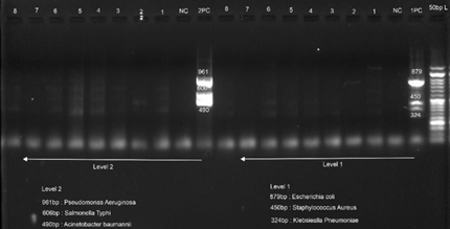
|
| Fig
1: Agarose gel image of Multiplex PCR
|
|
Table 7:
Association of asymptomatic
bacteriuria between Multiplex PCR
findings and Urine culture findings.
|
|
Growth detected
|
Total
|
|
Multiplex PCR
|
Urine culture
|
|
Growth positive
|
36
|
3
|
39
|
|
Growth negative
|
14
|
47
|
61
|
|
Total
|
50
|
50
|
100
|
|
Table 8:
Association of asymptomatic bacteriuria
between UHBP levels and Multiplex PCR
findings
|
|
UHBP levels (pg/ml)
|
Multiplex PCR findings
|
Total
|
|
culture +ve
|
Culture -ve
|
|
<870
|
8
|
4
|
12
|
|
>870
|
10
|
28
|
38
|
|
Total
|
18
|
32
|
50
|
|
Test: Fisher’s exact test
|
|
Table 9: Association of
asymptomatic bacteriuria between UHBP
levels and Urine culture
|
|
UHBP levels (pg/ml)
|
Urine Culture findings
|
Total
|
|
culture +ve
|
Culture -ve
|
|
<870
|
3
|
35
|
38
|
|
>870
|
0
|
12
|
12
|
|
Total
|
3
|
47
|
50
|
|
Test: Fisher’s exact test
Sensitivity: 0.7200, Specificity: 0.9400,
Positive Predictive Value: 0.9231,
Negative Predictive Value: 0.7705,
Likelihood ratio: 12.00 (Method used:
Wilson Brown)
|
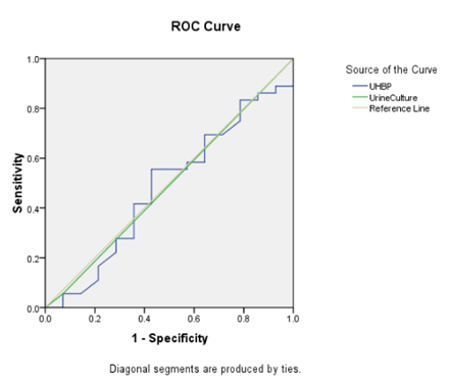
|
| Fig
2: ROC for Urine culture and UHBP, taking
multiplex PCR as gold standard |
AUC for the
Receiver operating characteristic curves (ROC) of
UHBP was 0.480 and that of urine culture was
0.492. Cut off value of UHBP was 675 pg/ml.
Discussion
Studies reported
the prevalence of ASB in GDM range from 4-18%
which is higher compared to the prevalence of ASB
in pregnancy without diabetes which range from
4.6-8.2%[10]. In our present study, the prevalence
of ASB in GDM as per urine culture was found to be
6%. The prevalence of ASB in GDM as per multiplex
PCR is higher compared to the urine culture.
Multiplex PCR also identified polymicrobial
infection (34%) accurately compared to urine
culture. Polymicrobial infection can result in
antibiotic resistance, therefore identification of
specific organism is of great clinical
significance [11]. Even though urine culture is
considered as the gold standard for the diagnosis
of ASB it takes 24-48 hours to get the results,
whereas PCR detected pathogens even in samples
which were negative for growth in urine culture in
a rapid and accurate way. As indicated in
Table 7,a significant association was
noted between detection of organisms and multiplex
PCR findings compared to urine culture (p
<0.0001). Out of 47 growth negative samples
from urine culture, whereas only 14 samples were
showed negative growth in multiplex PCR. This
further support the previously reported studies
that multiplex PCR can be used effectively to
detect UTI similar to urine culture and therefore
multiplex PCR is noninferior to urine culture for
detection of UTI [9,11].
Heparin-binding
protein (HBP) is a pro-inflammatory protein stored
in secretory and azurophilic granules of
neutrophils. Activated neutrophils release HBP
which acts as a chemoattractant, activates
monocytes and induces vascular leakage [12]. UHBP
was found to be more sensitive and specific for
UTI than the presence of leukocyturia and can be
used as a diagnostic marker for UTI in adults
[13]. However the potential of UHBP as a
diagnostic marker in ASB is not clear. In the
present study, UHBP levels in urine was noted to
be 689 (625, - 863) pg/ml. UHBP levels in urine
was significantly associated with multiplex PCR
results, but the association with urine culture
results was found to be non-significant. These
results further indicates that multiplex PCR
results are more specific compared to urine
culture results.
By taking multiplex
PCR as gold standard AUC for the ROC of UHBP was
0.480 and that of urine culture was 0.492. Cut off
value of UHBP was 675 pg/ml. These results suggest
the limitation of urine culture along with delay
in results. Multiplex PCR was found to be more
sensitive and specific compared to Urine culture
and estimation of UHBP.
In recent years,
researchers have been exploring the potential role
of urinary heparin binding protein (HBP) as a
biomarker for UTIs and inflammatory conditions.
HBP is a protein involved in the immune response
and is released by activated neutrophils, a type
of white blood cell, during inflammation. Its
presence in the urine may indicate inflammation
and tissue damage in the urinary tract. The
association between ASB/pyuria and urinary HBP in
the context of gestational diabetes is an area of
interest, as it could provide insights into the
pathophysiology of UTIs during pregnancy and
potentially lead to the development of better
diagnostic and management strategies.
A study published
by Mardh et al reported the association between
urinary HBP and ASB in pregnant women. The
researchers collected urine samples from pregnant
women with and without ASB and measured the levels
of urinary HBP using an ELISA-based assay. The
study found that pregnant women with ASB had
significantly higher levels of urinary HBP
compared to those without ASB, suggesting a
potential link between urinary HBP and the
presence of bacterial colonization in the urinary
tract during pregnancy[14].
Another study by
Cobo et al, examined the association between
urinary HBP and pyuria in pregnant women [15]. The
study found a positive correlation between the
levels of urinary HBP and the presence of pyuria,
indicating that HBP may be a potential biomarker
for inflammation and infection in the urinary
tract during pregnancy. (Reference:
Mardh et al have
shown variable sensitivities for UHBP in detecting
ASB, reporting a sensitivity of 84% for UHBP in
pregnant women with ASB [14].
Studies have shown
high sensitivities for multiplex PCR in detecting
ASB. For instance, A study Park et al reported a
sensitivity of 91% for multiplex PCR in detecting
ASB in a diverse population [16]. One drawback of
PCR-based methods is that they do not distinguish
between live and dead bacteria, potentially
leading to false positives in cases of past
infections or contamination.
Comparative studies
have been conducted to assess the diagnostic
performance of these three techniques. A study by
Jo et al compared the diagnostic accuracy of UHBP
measurement by ELISA and multiplex PCR for
detecting ASB in elderly patients [17]. The
researchers found that UHBP had a sensitivity of
81.5%, while multiplex PCR had a sensitivity of
93.4%. The specificity of UHBP and multiplex PCR
was 72.1% and 79.2%, respectively. The study
concluded that multiplex PCR had higher
sensitivity but lower specificity compared to UHBP
measurement.
Another study by
Mardh et al compared the diagnostic performance of
urine culture, UHBP measurement by ELISA, and
multiplex PCR in detecting ASB in pregnant
women[18]. The researchers reported a sensitivity
of 84% for UHBP measurement, 100% for multiplex
PCR, and 81% for urine culture. The specificities
for UHBP measurement, multiplex PCR, and urine
culture were 83%, 82%, and 100%, respectively. The
study suggested that UHBP measurement and
multiplex PCR had comparable sensitivities, but
urine culture had lower sensitivity (Reference:
Overall, multiplex
PCR appears to have higher sensitivity compared to
UHBP measurement and urine culture, making it a
promising technique for detecting ASB. However,
further research is needed to validate and compare
these methods in diverse populations and clinical
settings. Moreover, considering the cost,
availability, and turnaround time of each
technique is essential when choosing the most
suitable method for ASB detection.
Limitations
Limitations of the
existing research include relatively small sample
sizes, potential confounding factors, and the
cross-sectional nature of some studies, which
limits the ability to establish causality.
Additionally, the diagnostic accuracy and
threshold values for urinary HBP in detecting ASB
and pyuria need to be validated in larger and more
diverse populations.
Conclusion
In conclusion, the study suggest a potential
association between urinary HBP and ASB/pyuria in
gestational diabetes, more comprehensive research
is required to confirm and elucidate the nature of
this relationship. Future studies should focus on
larger cohorts, prospective designs, and
standardized measurement techniques to further
explore the role of urinary HBP as a potential
biomarker for UTIs during pregnancy and its
implications for gestational diabetes management.
Funding:
Research society for the study of diabetes in
India and Indian council of medical research.
References
- Metzger, BE. Long-term Outcomes in Mothers
Diagnosed With Gestational Diabetes Mellitus and
Their Offspring. Clinical Obstetrics and
Gynecology December 2007;50(4):972-979.
- Ferrara A, Kahn HS, Quesenberry CP, Riley C,
Hedderson MM. An increase in the incidence of
gestational diabetes mellitus: Northern
California, 1991-2000. Obstet Gynecol.
2004 Mar;103(3):526-33.
- Lee KW, Ching SM, Ramachandran V et al.
Prevalence and risk factors of gestational
diabetes mellitus in Asia: a systematic review
and meta-analysis. BMC Pregnancy Childbirth
2018;18:494.
- Choudhary N, Rasheed M, Aggarwal V. Prevalence
of gestational diabetes mellitus, maternal and
neonatal outcomes in a peripheral hospital in
North India. Int J Res Med Sci
2017;5:2343-5.
- Ghouri F, Hollywood A, Ryan K. A systematic
review of non-antibiotic measures for the
prevention of urinary tract infections in
pregnancy. BMC Pregnancy Childbirth.
2018 Apr 13;18(1):99.
- Rowe TA, Juthani-Mehta M. Urinary tract
infection in older adults. Aging Health. 2013
Oct;9(5):10.2217/ahe.13.38
- Renko M, Tapanainen P, Tossavainen P, Pokka T,
Uhari M. Meta-analysis of the significance of
asymptomatic bacteriuria in diabetes. Diabetes
Care. 2011 Jan;34(1):230-235.
- Schnarr J, Smaill F. Asymptomatic bacteriuria
and symptomatic urinary tract infections in
pregnancy. European Journal of Clinical
Investigation. 2008;38:50-57.
- Xu R, Deebel N, Casals R, Dutta R, Mirzazadeh
M. A New Gold Rush: A Review of Current and
Developing Diagnostic Tools for Urinary Tract
Infections. Diagnostics (Basel). 2021
Mar 9;11(3):479.
- Schnarr J, Smaill F. Asymptomatic bacteriuria
and symptomatic urinary tract infections in
pregnancy. Eur J Clin Invest. 2008
Oct;38 Suppl 2:50-7.
- Wojno KJ, Baunoch D, Luke N, et al. Multiplex
PCR Based Urinary Tract Infection (UTI) Analysis
Compared to Traditional Urine Culture in
Identifying Significant Pathogens in Symptomatic
Patients. Urology. 2020
Feb;136:119-126.
- Linder A, Soehnlein O, Akesson P. Roles of
heparin-binding protein in bacterial infections.
J Innate Immun. 2010;2(5):431-8.
- Kjölvmark C, Påhlman LI, Åkesson P, Linder A.
Heparin-binding protein: a diagnostic biomarker
of urinary tract infection in adults. Open
Forum Infect Dis. 2014 Apr
23;1(1):ofu004.
- Mårdh E, Brauner A, Söderquist B, et al.
Urinary heparin-binding protein in pregnant
women with asymptomatic bacteriuria. Eur J
Obstet Gynecol Reprod Biol. 2018
Feb;221:154-158. doi:
10.1016/j.ejogrb.2017.12.029.
- Cobo T, Rodríguez-Ortega M, Pérez-Bosque A, et
al. Association between pyuria and urinary
heparin-binding protein levels in pregnant women
with pre-gestational diabetes. Am J Reprod
Immunol. 2019 Oct;82(4):e13197. doi:
10.1111/aji.13197.)
- Park KH, Kim YJ, Jeong YJ, et al. Comparison
of the Urinary Microbiota Between Women with
Interstitial Cystitis and Healthy Controls. J
Clin Microbiol. 2015 Aug;53(8):2624-2631.
doi: 10.1128/JCM.00877-15.).
- Jo J, Lee K, Rhee JE, et al. Comparison of
diagnostic accuracy of urine dipstick and urine
heparin-binding protein in elderly patients with
asymptomatic bacteriuria. J Clin Microbiol.
2017 Jun;55(6):1865-1871. doi:
10.1128/JCM.00154-17.
- Mårdh E, Brauner A, Söderquist B, et al.
Urinary heparin-binding protein, a new
diagnostic marker for asymptomatic bacteriuria
in pregnant women: a multicentre cohort study. BMC
Infect Dis. 2016 Apr 12;16:198. doi:
10.1186/s12879-016-1546-4.
|



















