|
Introduction
Placenta
praevia (PP) is an obstetric complication
characterised by the presence of the placenta that
is covering the internal cervical os in the third
trimester. The cause of PP is unknown but there
are many known risk factors such as previous PP,
previous uterine surgery, multiparity etc. AS
Anzaku et al. (1) found prior history of CD as the
most common risk factor (40.7%). A recent WHO
publication reported that between 1990 and 2014
the global average CD rate increased from 12.4 to
18.6% with rates ranging, depending on region, (2)
and South African Saving Mothers reported a CD
rate of 28% in 2019, 2020 and 2021. There is a
well-known exponential increase in the risk of PP
with number of prior CD. (3)
The morbidity and
mortality of PP is increased when PP is associated
with PAS. Prior uterine surgery causes PAS with
prior CD as the most common predisposing factor,
(4-5) the decidua basalis abnormally
thin due to failed reconstruction following
disruption so the placenta can attach and invade
the myometrium. IM Usta et al. (6) found that
caesarean hysterectomy was performed only in women
with PAS who had a longer hospital stay, a higher
estimated blood loss, and need for blood
transfusion.
Queen Nandi Regional
Hospital (QNRH) in northern KwaZulu Natal has a
high prevalence of PP probably because of the wide
referral base. No studies on PP have been done
here. Hopefully this study will highlight the
clinical profile, complications and the
shortcomings in the management of PP.
Methods
The study was a
retrospective observational cross-sectional study
to review women’s medical records. The study was
conducted at QNRH which is a rural and regional
referral health facility for 16 district
hospitals. The study population comprised women
diagnosed during antenatal care with PP and
managed at the hospital from 1st
January 2018 to 31st December 2020. A
hospital register was used to identify women
diagnosed with PP. The hospital numbers were used
to retrieve women’s medical records. Through a
pre-designed structure data sheet, demographic
details and clinical information were obtained
including risk factors, medical and obstetric
history, ultrasound findings, gestational age at
diagnosis and delivery, management and
complications (antepartum, intrapartum and
post-partum). Only women with PP found on
ultrasound with a gestational age of ≥ 26 weeks
were included in the study.
Statistical analyses
were performed by using descriptive statistics.
Frequencies and percentages were used for
categorical data. Chi-Square-Test,
Mann–Whitney-Test, Fisher’s exact were used to
determine categorical factors associated with
outcome, applying a significance level α <
0.05, P values, odds ratios and 95 confidence
intervals were given.
The research was
approved by the Biomedical Research Ethics
Committee of the University of KwaZulu Natal
(BREC/00003226/2021), the KwaZulu Natal department
of Health (KZ _202111_002) and the ethic committee
of Queen Nandi Regional Hospital.
Results
Our study population
were women diagnosed with PP during the study
period, a sample size of 114 was required which
was calculated using Stata V15. Single women were
99 (86.8%), married women were 15 (13.2%),
employed were 25 (21.9%), unemployed were 76
(66.7%), students were 10 (8.8%), unknown
occupation were 3 (2.6%). Women with PP who
presented with complaint of per vagina bleeding
were 67 (58.8%). Women who booked before 14 weeks
gestational age were 46 (40.4%), 14- 22 weeks were
38 (33.3%), more than 22 weeks were 27 (23.7%) and
only 3 (2.6%) were unbooked. HIV negative were 63
(55.3%) and HIV positive were 51 (44.7%). Normal
BMI were 28 (24.6%), overweight were 28 (24.6%),
high BMI were 42 (36.8%) and 16 (14.0%) were
unknown. After diagnosis of PP was made, 87(76.3%)
were managed inside the hospital (asymptomatic or
symptomatic) and 27 (23.7%) were managed as
outpatient (asymptomatic). Emergency CD were 64
(56.1%). Regarding haemostasis management, 20
(17.2%) had uterine artery ligation, 1 (0.9%) had
internal iliac artery ligation, 17 (14.7%) had
compressive sutures, 9 (7.8%) had uterine
tamponade, 2 (1.7%) had stepwise
devascularisation, 4 (3.4%) had abdominal swabs
packed post TAH. All women with PAS (n=14) had a
prior CD and all had a total abdominal
hysterectomy, other cause of TAH was post-partum
haemorrhage (PPH) (n=3). No cell saver machine was
used for blood transfusion. General anaesthesia
was administered in 82 (71.9%) women with PP.
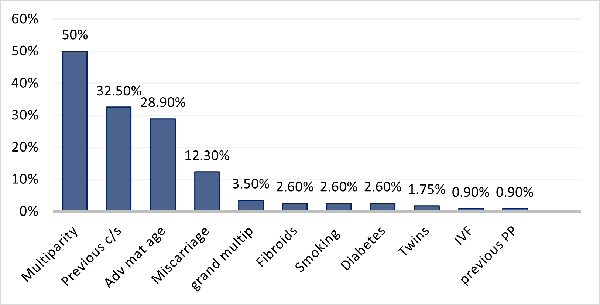
|
| Fig.
1: Risk factors of women with PP |
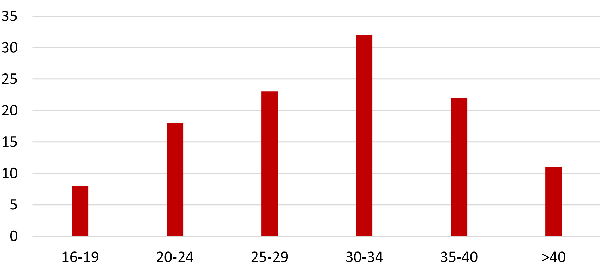
|
| Fig.
2: Maternal age distributions for women
with PP |
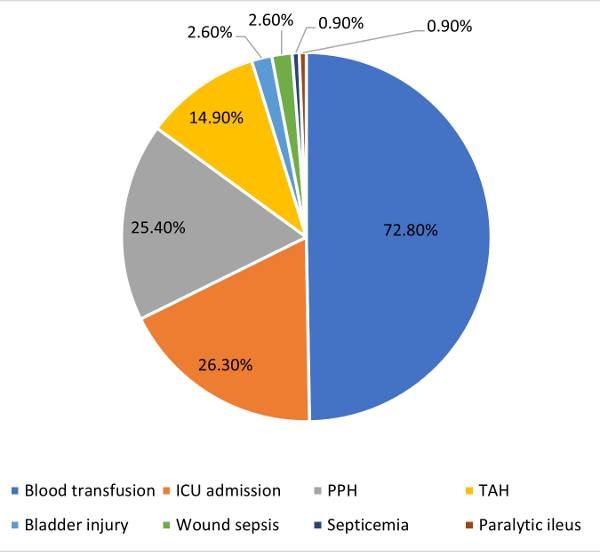
|
| Fig.
3: Complications of women with PP |
Discussion
This study showed
that most of participants (73.7%) first presented
at health facility before 22 weeks, high number
could be due to health initiative which aims to
support maternal health through the use of cell
phone based technologies integrated into maternal
and child health services. Most women in South
Africa (56%) do not attend health facility before
20 weeks due to numerous barriers such as
transportation, under-resourced clinics with
excessive waiting lines. (7)
The participants in
the study presented with different risk factors,
the most common including: multipara, prior CD and
increasing in maternal age (Fig. 1). The
Increasing in parity, documented to be 50% (table
1) as compared to 57% in study by M. Kollman et
al. (4) may be due to many unsettled
relationships, with single women found to be
86.8%, poor contraception uptake could also be the
reason.
|
Table 1: Obstetric history
|
|
Parity
|
N=114
|
%
|
|
P0
|
15
|
13.2
|
|
P1
|
38
|
33.3
|
|
P2-4
|
57
|
50.0
|
|
≥5
|
4
|
3.5
|
|
Previous mode of delivery
|
N=114
|
%
|
|
NVD
|
77
|
67.5
|
|
Previous CD x1
|
18
|
15.8
|
|
Previous CD x2
|
12
|
10.5
|
|
Previous CD x3 or more
|
7
|
6.1
|
The high prevalence
of prior CD (32.5%) could be the result of
increased CD rate worldwide, (2) and a wide
referral base at QNRH, AS Anzaku et al. (1) and E.
Jaiswal et al. (8) found respectively a prior CD
rate of 40.7% and 35.4%. Surgical disruption of
the uterine cavity is known to cause lasting
damage to the myometrium and endometrium and the
risk of PP is increased with the increased number
of prior CD. (3)
Advanced maternal
age was 28.9% (Fig. 1 and 2), same number (29.3%)
was found by M. Kollman et al. (4) According
to the study conducted by Choi et al. (9) prevalence
of PP increases as the maternal age advances. This
is thought to be due to atherosclerotic changes in
the uterus resulting in under perfusion and
infraction of the placenta, thereby increasing the
size of the placenta.
A low lying placenta
may be found as soon as 18-20 weeks gestational
age when an ultrasound is done for an anomaly
ultrasound but the diagnosis of PP will needs to
be confirmed at around 32 weeks as 90% of PP will
resolve before delivery at term. (10) Early
access to ultrasound seemed to be limited or
delayed in the participants as only 37.7% had
their first ultrasound before 28 weeks gestational
age (Table 2). For women who didn’t have an
ultrasound done early in pregnancy, the diagnosis
may be suspected and confirmed when they present
with per vagina bleeding, this study found 58.8%
of participants presented with per vagina
bleeding.
|
Table 2: Gestational age at
diagnosis and delivery
|
|
Gestational age at diagnosis
(weeks)
|
N
|
%
|
|
<28
|
43
|
37.7
|
|
28-33
|
44
|
38.6
|
|
34-36
|
15
|
13.2
|
|
≥37
|
12
|
10.5
|
|
Total
|
114
|
100
|
|
Gestational age at Delivery
(weeks)
|
N
|
%
|
|
28-33
|
30
|
26.3
|
|
34-36
|
33
|
28.9
|
|
≥37
|
51
|
44.7
|
|
Total
|
114
|
100
|
Per vagina bleeding
is the main reason for early delivery with 56.1%
of participants who required emergency CD. E.
Jaiswal et al. (8) found 81.5% with per vagina
bleeding, with 70.8% who had emergency CD. The
bleeding can be very massive, putting the life of
the woman and her pregnancy in danger, emphasizing
the importance of early access to ultrasound and
early diagnosis.
The detection rate
of PAS was low as only 5 out 14 (35.7%) (Table 3)
were diagnosed by ultrasound with the remainder
diagnosis made intra operatively after failed
separation of the placenta. G Pagani et al. (11)
found a sensitivity of 88% on sonographic
identification of PAS. The combination of
grey-scale and colour Doppler imaging ultrasound
markers is reported to have increased the
sensitivity of ultrasound imaging to around 90%
with negative predictive values ranging between
95% and 98%. (12)
|
Table 3: Ultrasound findings at
diagnosis
|
|
Placenta praevia classification
|
N=114
|
%
|
|
Minor
|
43
|
37.7
|
|
Major
|
71
|
62.3
|
|
Placenta praevia location
|
N=114
|
%
|
|
Anterior
|
49
|
42.9
|
|
Posterior
|
53
|
46.4
|
|
Antero-posterior
|
7
|
6.1
|
|
Lateral
|
5
|
4.4
|
|
Signs of morbid adherence
|
N=14
|
%
|
|
Yes
|
5
|
35.7
|
|
No
|
9
|
64.3
|
Most of participants
were managed as inpatients (76.3%), many of them
came from far with limited access to
transportation or were at high risk of bleeding
and needed to be managed inside the hospital.
Comparison of outcome between inpatient and
outpatient management was not done, due to
insufficient medical notes but Wing et al. (13)
found no difference in outcomes between inpatient
and outpatient management in his randomized
control trial.
Intra operatively,
the main concern is excessive bleeding. The most
common fertility sparing technique used to achieve
haemostasis in the study was uterine artery
ligation (17.2%) while in study by E. Jaiswal et
al. (8) placenta bed compressive sutures were
mostly used (39.2%). A combination of techniques
can be used to achieve haemostasis if one isn’t
successful and because bleeding comes from the
placenta bed, compressive sutures and uterine
tamponade may be among the first to be done.
Most of participants
received general anaesthesia (71.9%), probably due
to the perception that it is safer especially for
major degree PP or PP associated with PAS. JY Hong
et al. (14) compared general and regional
anaesthesia (RA) found the latter was more
superior in elective caesarean section for major
degree PP with regard to maternal haemodynamic and
blood loss but there was no difference in the
incidence of intraoperative or anaesthesia
complications between the 2 types of anaesthesia.
The study found
different complications associated with PP. (Fig.
3) The study found 25.4% of participants had PPH
(table 4), same number (24.6%) found by E. Jaiswal
et al. (8) Lower segment of the uterus
contracts poorly leading to excessive bleeding in
women with major degree PP. In this study, the
risk of PPH increased with the number of prior CD
(5 fold Odds increase with Prior CDx3) (Table 5),
J. Hasegawa et al. (15) had the same observation.
This may be due to prior CD that is associated
with PAS due to an absence or deficiency of
spongiosus layer of the decidua and the main risk
associated with any form of PAS disorder is
massive obstetric haemorrhage, (16) with result
found to be statistically significant in this
study. (Table 5)
|
Table 4: Number of blood units
transfused and estimated blood loss
|
|
Unit of blood transfusion
|
N
|
%
|
|
0
|
31
|
27.2
|
|
1-2
|
48
|
42.1
|
|
3-4
|
18
|
15.8
|
|
5-6
|
12
|
10.5
|
|
7-8
|
5
|
4.4
|
|
Total
|
114
|
100
|
|
Intraoperative estimated blood
loss
|
N
|
%
|
|
<500 ml
|
28
|
24.6
|
|
500-900ml
|
47
|
41.2
|
|
≥1000 ml
|
29
|
25.4
|
|
Unknown
|
10
|
8.7
|
|
Total
|
114
|
100
|
|
Table 5: Postpartum haemorrhage
compared with maternal characteristics
|
|
Postpartum haemorrhage
|
|
Yes
|
No
|
|
|
|
|
|
Variable
|
n
|
n
|
t
|
p
|
OR
|
95 CI
|
|
Grading of PP
|
|
|
|
|
|
|
|
Minor
|
4
|
39
|
43
|
0.002
|
|
|
|
Major
|
25
|
46
|
71
|
|
5.30
|
1.70-16.54
|
|
Total
|
29
|
85
|
114
|
|
|
|
|
Prior delivery
|
|
|
|
|
|
|
|
NVD
|
15
|
62
|
77
|
0.02
|
|
|
|
CDx1
|
6
|
12
|
18
|
|
2.07
|
0.67-6.40
|
|
CDx2
|
4
|
8
|
12
|
|
2.07
|
0.55-7.78
|
|
CDx3
|
4
|
3
|
7
|
|
5.51
|
1.11-27.29
|
|
Total
|
29
|
85
|
114
|
|
|
|
|
PAS
|
|
|
|
|
|
|
|
Yes
|
10
|
4
|
14
|
<0.001
|
|
|
|
No
|
19
|
81
|
100
|
|
0.09
|
0.03-0.33
|
|
Total
|
29
|
85
|
114
|
|
|
|
|
Parity
|
|
|
|
|
|
|
|
P0
|
5
|
10
|
15
|
0.78
|
|
|
|
P1
|
8
|
30
|
38
|
|
0.53
|
0.14-2.01
|
|
P2-4
|
12
|
45
|
57
|
|
0.78
|
0.23-2.64
|
|
≥5
|
4
|
0
|
4
|
|
|
|
|
Total
|
29
|
85
|
114
|
|
|
|
The study found that
the risk of TAH increased with the number of prior
CD, probably due to the association of prior CD
and PAS. All participants diagnosed with PAS had a
TAH. (Table 6) IM Usta et al. (6) and DA Miller et
al. (17) found also an increased risk of PAS with
number of prior CD. There is a place for a
conservative management (leaving the placenta in
situ for spontaneous resorption) when fertility is
still desired, (18) no participant with PAS in
this study was managed conservatively as all had
completed family and were agreeable for TAH after
counselling.
|
Table 6: Hysterectomy compared
with maternal characteristics
|
|
Hysterectomy
|
|
Yes
|
No
|
|
|
|
|
|
Variable
|
n
|
n
|
t
|
p
|
OR
|
95 CI
|
|
Grading of PP
|
|
|
|
|
|
|
|
Minor
|
1
|
42
|
43
|
0.005
|
|
|
|
Major
|
16
|
55
|
71
|
|
11.25
|
1.43-88.57
|
|
Total
|
17
|
97
|
114
|
|
|
|
|
Prior delivery
|
|
|
|
|
|
|
|
NVD
|
1
|
76
|
77
|
<0.001
|
|
|
|
CD x1
|
3
|
15
|
18
|
|
15.20
|
1.48-156.21
|
|
CD x2
|
7
|
5
|
12
|
|
106.40
|
10.86-1042.68
|
|
CD x3
|
6
|
1
|
7
|
|
190.00
|
14.61-2470.99
|
|
Total
|
17
|
97
|
114
|
|
|
|
|
PAS
|
|
|
|
|
|
|
|
Yes
|
14
|
0
|
14
|
<0.001
|
|
|
|
No
|
3
|
97
|
100
|
|
0.002
|
0.000-0.021
|
|
Total
|
17
|
97
|
114
|
|
|
|
|
Parity
|
|
|
|
|
|
|
|
P0
|
0
|
14
|
14
|
0.06
|
|
|
|
P1
|
3
|
36
|
39
|
|
0.78
|
0.07-9.27
|
|
P2-4
|
10
|
47
|
57
|
|
4.14
|
0.50-34.50
|
|
≥5
|
4
|
0
|
4
|
|
|
|
|
Total
|
17
|
97
|
114
|
|
|
|
Majority of
participants (72.8%) in the study received blood
transfusion. (Fig. 3) The risk of blood
transfusion increased with prior CD (5 fold Odds
increase with prior CDx2) (Table 7), WA Grobman et
al. (19) had the same observation but
A Oya et al. (20) found increased risk of blood
transfusion only in major degree PP and not in
prior CD, difference could be due to small number
of prior CD in his study. Our study showed that no
cell saver machine was used even though it was
available. S Malik et al. (21) found the usage of
cell salvage practice was not very effective due
to non-availability of trained staff and
unfamiliarity of techniques, explanation may be
similar in this study, but S Malik concluded that
if it is used efficiently it has a role in
decreasing the need for homologous blood
transfusion and saving cost.
|
Table 7: Blood transfusion
compared with maternal characteristics
|
|
Blood transfusion
|
|
Yes
|
No
|
|
|
|
|
|
Variable
|
n
|
n
|
t
|
p
|
OR
|
95 CI
|
|
Classification
|
|
|
|
|
|
|
|
Minor
|
26
|
17
|
43
|
0.03
|
|
|
|
Major
|
57
|
14
|
71
|
|
2.66
|
1.14-6.20
|
|
Total
|
83
|
31
|
114
|
|
|
|
|
Parity
|
|
|
|
|
|
|
|
P0
|
10
|
5
|
15
|
0.01
|
|
|
|
P1
|
22
|
16
|
38
|
|
0.69
|
0.20-2.40
|
|
P2-4
|
47
|
10
|
57
|
|
2.35
|
0.66-8.39
|
|
≥5
|
4
|
0
|
4
|
|
|
|
|
Total
|
83
|
31
|
114
|
|
|
|
|
Prior delivery
|
|
|
|
|
|
|
|
NVD
|
50
|
27
|
77
|
0.005
|
|
|
|
CD x1
|
15
|
3
|
18
|
|
2.70
|
0.72-10.6
|
|
CD x2
|
11
|
1
|
12
|
|
5.90
|
0.73-48.50
|
|
≥ CD x3
|
7
|
0
|
7
|
|
|
|
|
Total
|
83
|
31
|
114
|
|
|
|
|
PAS
|
|
|
|
|
|
|
|
Yes
|
14
|
0.0
|
14
|
0.01
|
|
|
|
No
|
69
|
31
|
100
|
|
|
|
|
Total
|
83
|
31
|
114
|
|
|
|
Placenta praevia is
associated with a high risk of preterm delivery,
mainly due to antepartum haemorrhage. (22) This
study found 26.3% of participants delivered before
34 weeks gestational age (Table 3) as compared to
41.5% by E. Jaiswal et al. (8) Participants who
delivered before 37 weeks were 55.3% as compared
to 45.6% by JM Crane et al. (23) Prematurity
may explain other neonate adverse outcomes found
in the study such as, respiratory problems, low
APGAR and perinatal death (Table 8 and 9).
Respiratory problems were more likely in smaller
babies as compared to bigger babies (Table 10),
steroid for lung maturity was not included in the
study due to insufficient notes, if not given can
explain the respiratory problems in small babies.
|
Table 8: Neonates’ outcome
|
|
Fetal birth condition
|
n
|
%
|
|
Alive good APGAR
|
99
|
85.3
|
|
Alive with low APGAR
|
11
|
9.5
|
|
FSB
|
2
|
1.7
|
|
MSB
|
1
|
0.9
|
|
ENND
|
3
|
2.6
|
|
Total
|
116
|
100
|
|
Birth weight
|
n
|
%
|
|
<1500
|
15
|
12.9
|
|
1500-1999
|
15
|
12.9
|
|
2000-2499
|
18
|
15.5
|
|
2500-3000
|
43
|
37.1
|
|
>3000
|
25
|
21.6
|
|
Total
|
116
|
100
|
|
Baby sex
|
n
|
%
|
|
Male
|
63
|
54.3
|
|
Female
|
53
|
45.7
|
|
Total
|
116
|
100
|
|
NICU/Nursery stay
|
n
|
%
|
|
Yes
|
39
|
33.6
|
|
No
|
77
|
66.4
|
|
Total
|
116
|
100
|
|
Table 9: Reason for neonate
admission to nursery
|
|
Respiratory problems (RDS, HMD,
TTN)
|
n
|
%
|
|
Yes
|
23
|
19.8
|
|
No
|
93
|
80.2
|
|
Total
|
116
|
100
|
|
Birth weight ≤1500g
|
n
|
%
|
|
Yes
|
15
|
12.9
|
|
No
|
101
|
87.1
|
|
Total
|
116
|
100
|
|
Other reasons
|
n
|
%
|
|
Yes
|
14
|
12.1
|
|
No
|
102
|
87.9
|
|
Total
|
116
|
100
|
|
Unknown
|
n
|
%
|
|
Yes
|
4
|
3.4
|
|
No
|
112
|
96.6
|
|
Total
|
116
|
100
|
|
Table 10: Neonates’ respiratory
problems compared with foetal birth
weight
|
|
Respiratory problems (TTN, RDS,
HMD)
|
|
Yes
|
No
|
|
|
|
|
|
Variable
|
n
|
n
|
t
|
p
|
OR
|
95 CI
|
|
Birth weight
|
|
|
|
|
|
|
|
<1500
|
6
|
9
|
15
|
<0.001
|
1.71
|
0.40-7.29
|
|
1500-1999
|
8
|
7
|
15
|
|
0.58
|
0.13-2.48
|
|
2000-2499
|
5
|
13
|
18
|
|
0.07
|
0.01-0.42
|
|
2500-3000
|
2
|
41
|
43
|
|
0.13
|
0.02-0.77
|
|
>3000
|
2
|
23
|
25
|
|
|
|
|
Total
|
23
|
93
|
116
|
|
|
|
This study being the
first done at QNRH, add to the body of evidence
the morbidity associated with PP. Future
researchers may use this study as a reference for
further studies. Clinicians may also refer to this
study to better understand the condition.
Limitations and strengths
This was a
retrospective study with recordkeeping not optimal
in some cases. There were no maternal deaths. This
study is the first in this rural health
institution, identified maternal risk factors
associated with bad outcome and findings could be
used to improve management of women with PP.
Conclusion
Prior history of CD
is a common risk factor for PP, when associated
with PAS, there is an increased risk of
complications such as TAH, PPH and blood
transfusion. Prematurity is a major concern in
women with PP. Limiting the number of caesarean
section in future will decrease the incidence of
PP and its complications. Further research may
involve outcome of women with PP associated with
PAS and manage conservatively by leaving the
placenta in situ.
Acknowledgements: The
authors thank all those who support this study, Dr
N. Mayat, Dr P. Makinga, Dr Motsema, Sister
Tshangase, and Catherine Conneli.
References
- Anzaku A, Musa J. Placenta praevia: incidence,
risk factors, maternal and foetal outcomes in a
Nigerian teaching hospital. Jos Journal of
Medicine. 2012;6(1): p. 42-46.
https://doi.org/10.3109/14767058.2015.1049152
- Harrison MS, Goldenberg RL. Caesarean section
in sub-Saharan Africa. Maternal Health,
Neonatology and Perinatology.
2016;2(1):1-10.
https://doi.org/10.1186/s40748-016-0033-x
- Ananth CV, Smulian JC, Vintzileos AM. The
association of placenta praevia with history of
caesarean delivery and abortion: a
meta-analysis. American Journal of
Obstetrics and Gynaecology.
1997;177(5):1071-1078.
https://doi.org/10.1016/s0002-9378 (97)70017-6
- Kollmann M, Gaulhofer J, Lang U et al.
Placenta praevia: incidence, risk factors and
outcome. The Journal of Maternal-Foetal and
Neonatal Medicine. 2016;29(9):1395-1398.
https://doi.org/10.3109/14767058.2015.1049152
- Matsuzaki S, Mandelbaum RS, Sangara RN, et al.
Trends, characteristics, and outcomes of
placenta accreta spectrum: a national study in
the United States. American Journal of
Obstetrics and Gynaecology.
2021;225(5):534. e1-534. e38.
https://doi.org/10.1016/j.ajog.2021.04.233
- Usta IM, Hobeika EM, Musa AA et al. Placenta
praevia-accreta: risk factors and complications.
American Journal of Obstetrics and
Gynaecology. 2005;193(3):1045-1049.
https://doi.org/10.1016/j.ajog.2005.06.037
- Haddad DN, Makin JD, Pattinson RC et al.
Barriers to early prenatal care in South Africa.
International Journal of Gynaecology and
Obstetrics. 2016;132(1):64-67.
https://doi.org/10.1016/j.ijgo.2015.06.041
- Jaiswal E, Aggarwal N, Suri V et al. Clinical
profile and outcome of patients with placenta
praevia: a study at a tertiary care referral
institute in Northern India. International
Journal of Reproduction, Contraception,
Obstetrics and Gynaecology.
2018;7(7):2560.
https://doi.org/10.18203/2320-1770.ijrcog20182861
- Choi SJ, Song SE, Jung KL et al. Antepartum
risk factors associated with peripartum
caesarean hysterectomy in women with placenta
praevia. American Journal of Perinatology.
2007;37-41.
https://doi.org/10.1055/s-2007-1004834
- Oyelese Y, Smulian JC. Placenta praevia,
placenta accreta, and vasa praevia. Obstetrics
and Gynaecology. 2006;107(4):927-941.
https://doi.org/10.1097/01.aog.0000252288.13209.40
- Pagani G, Cali G, Acharya G, et al. Diagnostic
accuracy of ultrasound in detecting the severity
of abnormally invasive placentation: a
systematic review and meta‐analysis. Acta
Obstetricia et Gynecologica Scandinavica.
2018;97(1):25-37.
https://doi.org/10.1111/aogs.13238
- D'Antonio F, Iacovella C, Bhide A. Prenatal
identification of invasive placentation using
ultrasound: systematic review and meta‐analysis.
Ultrasound in Obstetrics and Gynaecology.
2013;42(5):509-517.
https://doi.org/10.1002/uog.13194
- Wing DA, Paul RH, Millar LK. Management of the
symptomatic placenta praevia: a randomized,
controlled trial of inpatient versus outpatient
expectant management. American Journal of
Obstetrics and Gynaecology.
1996;175(4):806-811.
https://doi.org/10.1016/s0002-9378 (96)80003-2
- Hong JY, Jee YS, Yoon HJ et al. Comparison of
general and epidural anaesthesia in elective
caesarean section for placenta praevia totalis:
maternal hemodynamic, blood loss and neonatal
outcome. International Journal of Obstetric
Anaesthesia. 2003;12(1):12-16.
https://doi.org/10.1016/s0959-289x (02)00183-8
- Hasegawa J, Matsuoka R, Ichizuka K, et al.
Predisposing factors for massive haemorrhage
during caesarean section in patients with
placenta praevia. Ultrasound in Obstetrics
and Gynaecology. 2009;34(1):80-84.
https://doi.org/10.1002/uog.6426
- Allen L, Jauniaux E, Hobson S et al. FIGO
consensus guidelines on placenta accreta
spectrum disorders: nonconservative surgical
management. International Journal of
Gynaecology and Obstetrics. 2018.
https://doi.org/10.1002/ijgo.12409
- Miller DA, Chollet JA, Goodwin TM. Clinical
risk factors for placenta praevia–placenta
accreta. American Journal of Obstetrics and
Gynaecology. 1997;177(1):210-214.
https://doi.org/10.1016/s0002-9378 (97)70463-0
- Sentilhes L, Ambroselli C, Kayem G, et al.
Maternal outcome after conservative treatment of
placenta accreta. Obstetrics and
Gynaecology. 2010;115(3):526-534.
https://doi.org/10.1097/aog.0b013e3181f738d8
- Grobman WA, Gersnoviez R, Landon MB, et al.
Pregnancy outcomes for women with placenta
praevia in relation to the number of prior
caesarean deliveries. Obstetrics and
Gynecology. 2007;110(6):1249-1255.
https://doi.org/10.1016/j.ajog.2006.10.415
- Oya A, Nakai A, Miyake H et al. Risk factors
for peripartum blood transfusion in women with
placenta praevia: a retrospective analysis. Journal
of Nippon Medical School.
2008;75(3):146-151.
https://doi.org/10.1272/jnms.75.146
- Malik S, Brooks H, Singhal T. Cell saver use
in obstetrics. Journal of Obstetrics and
Gynaecology. 2010;30(8):826-828.
https://doi.org/10.3109/01443615.2010.511727
- Long SY, Yang Q, Chi R et al. Maternal and
neonatal outcomes resulting from antepartum
haemorrhage in women with placenta praevia and
its associated risk factors: A single-centre
retrospective study. Therapeutics and
Clinical Risk Management. 2021;31-38.
https://doi.org/10.2147/tcrm.s288461
- Crane JM, Van den Hof MC, Dodds L et al.
Neonatal outcomes with placenta praevia. Obstetrics
and Gynecology. 1999;93(4):541-544.
https://doi.org/10.1016/s0029-7844 (98)00480-3
|



















