|
Introduction
Tardive
Dyskinesia is a drug-induced hyperkinetic movement
disorder that is precipitated due to prolonged
exposure to dopamine receptor-blocking agents,
usually antipsychotics, which persists for minimum
30 days after stopping the drug (1). This
condition can be non-reversible and might last a
whole lifetime. The condition can be stigmatizing
and disabling, with detrimental effects on mental
and physical health and quality of life.
The term "tardive,"
or late, differentiates tardive dyskinesia from
various drug-induced extrapyramidal symptoms that
usually appear either acutely or very soon after
exposure to dopamine receptor-blocking agents and
that resolve after the drug is discontinued.
Tardive dyskinesia
(TD) is characterized by choreiform, athetoid, and
rhythmic abnormal involuntary movements.
The estimated annual
incidence of TD with first-generation
antipsychotics is 5% to 6% overall (2,3) and 10%
to 25% in adults(4,5). The risk is similar among
various first-generation antipsychotics when used
as dose equivalents.
The estimated annual
risk of tardive dyskinesia with exposure to
second-generation antipsychotics is approximately
4% in the population(6) and 5% to 7% in older
adults(7).
Tardive dyskinesia
is an important marker for patients at risk of
adverse healthcare outcomes and diminished quality
of life.
With a rise access
to psychiatric services and use of antipsychotics,
the need to study tardive dyskinesia becomes
important. The current global prevalence is
believed to be between 15-50% but the true
prevalence is not well studied, especially in the
Indian context.(8)
This study will help
in knowing the extent of tardive dyskinesia among
persons with chronic mental illness in a
Psychiatry Rehabilitation setting in India and
will also give us insights to the factors involved
to help prevent the same.
Materials and Methods:
The present study
was carried out between January 1st, 2021 and
April 2022 in the following rehabilitation centres
in Dakshina Kannada district, after obtaining the
clearance from Institution’s Ethics Committee
(YEC2/658).
- Swadhara women’s home Jeppu, Mangalore.
- Snehalaya asharam, Manjeshwara.
Study
Design: Descriptive type of
observational study, cross-sectional study
Assessment
Tools:
Abnormal
Involuntary Movement Scale (AIMS)
The AIMS(9) is a 12
items clinician-rated scale to assess the severity
of dyskinesia in patients taking antipsychotic
medications. Additional items are present to
assess the overall severity, incapacitation, and
the subject’s level of awareness of the movements,
and distress associated with them.
The AIMS has been
used extensively to assess Tardive Dyskinesia in
clinical trials of antipsychotic medications. Due
to its simple design and low assessment time, the
AIMS can be done regularly and effectively by all
clinicians.
Items are scored on
a 0 (none) to 4 (severe) basis. The scale provides
a total score or item 8 can be used in isolation
as an indication of the overall severity of
symptoms.
Kane Schooler
Criteria
It is a diagnostic
criterion(10) for tardive dyskinesia that has
three components and makes use of the AIMS scale
to do so. The components of this criteria are-
- At least 3 months of cumulative exposure to
neuroleptics (antipsychotics).
- Absence of other conditions that might cause
involuntary movements
- At least moderate dyskinetic movements in one
body area (≥ 3 on AIMS) or mild dyskinetic
movements in two body areas (≥ 2 on AIMS)
Sources of
Data/ Sampling Method: Inhabitants of
Swadhara women’s home Jeppu and Snehalaya asharam
who fulfilled the inclusion criteria were
recruited for this study. Written permission was
sought from the Rehabilitation center incharge and
the treating team. Informed consent was then
obtained from all the participants.
Type of sampling:
Convenience sampling
Sample Size
From the published
article, “Prevalence and risk factors associated
with tardive dyskinesia among Indian patients with
schizophrenia” by Rashmin M. Achalia(11) at 5%
level of significance and anticipated prevalence
was 26.4% (from related article) and estimation
error (absolute precision) of 6%
Sample size
calculation was done using the formula, n= [Z2Xp(1-p)]/d2
where, n= sample size d=absolute precision
p=prevalence. Total sample size was found to be
207.
Inclusion
Criteria:
- Patients of ages 18 or above
- Males/ females
- Patients with chronic mental illness currently
on antipsychotics for a minimum period of 3
months duration
Exclusion
Criteria:
- Patients unfit for a formal examination
- Patients with active symptoms of present
mental illness
- Subnormal intelligence clinically
The authors were not
directly involved in the treatment of all these
patients; as they were managed by their primary
physicians and psychiatrists.
Statistical
Analysis:
The data was entered
into a MS-Excel worksheet and cleaned for any
corrections and errors. Further analysis of data
was done using statistical package IBM SPSS
Statistics 26.0.
The nominal and
ordinal variables were presented using frequency
and percentages. The ratio scale variables were
presented using descriptive statistics such as
Mean and SD. Further analysis was done using
Chi-square test and unpaired t-test. The level of
significance was set at 5%. All p-values less than
0.05 were treated as significant.
Results:
Table 1 describes
the sociodemographic variables.
The average age of
study subjects was 45.39 years (SD=12.6336), and
the median age was 47.0 years and modal age was
55.0. The minimum and maximum age was 18 and 74
respectively.
Out of 207 study
subjects, the 139 (67.10%) had psychosis
unspecified/ schizophrenia and 68 (32.90%) had
bipolar disorder. Out of 207 study subjects, 45
(21.73%) had used anticholinergics.
|
Table 1: Sociodemographic data
|
|
Sociodemographic variables
|
Frequency
|
Percentage
|
|
1. Age (years)
|
|
|
|
18-30
|
38
|
18.35
|
|
31-40
|
33
|
15.94
|
|
41-50
|
56
|
27.05
|
|
51-60
|
62
|
29.95
|
|
>60
|
18
|
8.69
|
|
Total
|
207
|
100.00
|
|
2. Duration of illness (Years)
|
|
|
|
0-10
|
56
|
27.05
|
|
10-20
|
65
|
31.40
|
|
20-30
|
56
|
27.05
|
|
30-40
|
22
|
10.62
|
|
40-50
|
8
|
3.86
|
|
Total
|
207
|
100.00
|
|
3. Family history
|
|
|
|
Yes
|
16
|
7.72
|
|
No
|
191
|
92.27
|
|
Total
|
207
|
100.00
|
|
4. ECT received
|
|
|
|
Yes
|
20
|
9.66
|
|
No
|
187
|
90.33
|
|
Total
|
207
|
100.00
|
|
5. Anticholinergic drugs
|
|
|
|
No
|
162
|
78.26
|
|
Yes
|
45
|
21.73
|
|
Total
|
207
|
100.00
|
|
6. Place of residence
|
|
|
|
Urban
|
96
|
46.37
|
|
Rural
|
111
|
53.62
|
|
Total
|
207
|
100.00
|
|
7. Education
|
|
|
|
Illiterate
|
148
|
71.49
|
|
Primary
|
36
|
17.39
|
|
10th Pass
|
16
|
7.72
|
|
12th Pass
|
6
|
2.89
|
|
Graduate
|
1
|
0.48
|
|
Total
|
207
|
100.00
|
|
8. Marital status
|
|
|
|
Single
|
34
|
16.42
|
|
Married
|
149
|
71.98
|
|
Divorced
|
5
|
2.41
|
|
Separated
|
8
|
3.86
|
|
Widowed
|
11
|
5.31
|
|
Total
|
207
|
100.00
|
|
9. Socio-economic status
|
|
|
|
Above poverty line
|
54
|
26.08
|
|
Below poverty line
|
153
|
73.91
|
|
Total
|
207
|
100.00
|
Figure 1 indicates that 67% participants
were diagnosed with schizophrenia and 33% were
diagnosed with bipolar disorder.

|
Fig.
1: Diagnosis among participants (N=207)
|
Table 2 indicates distribution of study subjects
according to the medicines used.
|
Table 2: Distribution according to
medicines used
|
|
Medicine
|
Frequency
|
Percentage
|
|
Risperidone
|
116
|
56.03%
|
|
Olanzapine
|
68
|
32.85%
|
|
Amisulpride
|
40
|
19.32%
|
|
Quetiapine
|
23
|
11.11%
|
|
Cariprazine
|
14
|
6.76%
|
|
Clozapine
|
14
|
6.76%
|
|
Haloperidol
|
9
|
4.34%
|
|
| Fig.
2: Duration of treatment |
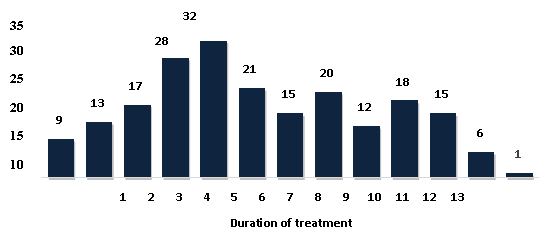
Figure 2 depicts the
distribution of subjects according to the duration
of treatment.
Table 3 indicates
distribution of study subjects according to the
CPZ equivalent doses/day for current ongoing
antipsychotics.
| Table
3: Chlorpromazine Cumulative Equivalent Dose |
| Chlorpromazine Cumulative Equivalent
Dose |
Frequency |
Percent |
|
50
|
9
|
4.34
|
|
100
|
9
|
4.34
|
|
125
|
1
|
0.48
|
|
150
|
4
|
1.93
|
|
200
|
79
|
38.16
|
|
225
|
1
|
0.48
|
|
250
|
17
|
8.21
|
|
275
|
1
|
0.48
|
|
300
|
40
|
19.32
|
|
325
|
1
|
0.48
|
|
350
|
15
|
7.24
|
|
400
|
19
|
9.17
|
|
450
|
6
|
2.89
|
|
500
|
2
|
0.96
|
|
550
|
1
|
0.48
|
|
600
|
1
|
0.48
|
|
650
|
1
|
0.48
|
|
Total
|
207
|
100.00
|
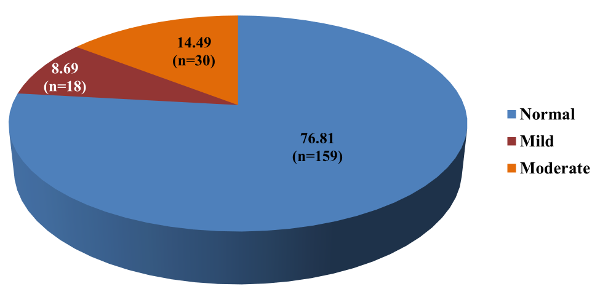
Figure 3 indicates
distribution of study subjects according to the
AIMS- facial and oral score. Out of 207 study
subjects, 18 (8.69%) had mild Tardive Dyskinesia
and 30 (14.49%) had moderate Tardive Dyskinesia.
|
Fig.
3: AIMS - facial and oral (N=207)
|
Overall prevalence
of Tardive Dyskinesia according to AIMS (facial
and oral) was 23.2%.
According to AIMS
(extremity) and AIMS (trunk) scores, none of the
207 subjects had TD.
Table 4 indicates
distribution of study subjects according to the
AIMS- global score and distribution of study
subjects according to the Schooler Kane criteria.
Out of 207 study subjects, 13 (6.28%) had mild
Tardive Dyskinesia and 20 (9.66%) had moderate
Tardive Dyskinesia.
Overall prevalence
of Tardive Dyskinesia according to AIMS (global)
score was 16%
Out of 207 study
subjects, 31 (14.5%) had mild Tardive Dyskinesia.
Overall prevalence of Tardive Dyskinesia according
to Schooler Kane criteria was 14.5%.
|
Table 4: AIMS - Global
|
|
Frequency
|
Percent
|
|
Normal
|
174
|
84.05
|
|
Mild
|
13
|
6.28
|
|
Moderate
|
20
|
9.66
|
|
Total
|
207
|
100.00
|
|
Schooler Kane criteria satisfied
|
|
Satisfied
|
31
|
14.49
|
|
Not satisfied
|
177
|
85.50
|
|
Total
|
207
|
100.00
|
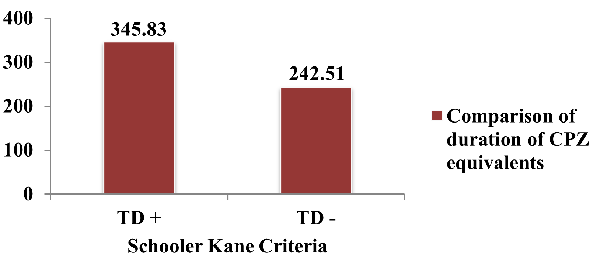
Figure 4 depicts
comparison of CPZ equivalent doses/day for current
ongoing antipsychotics according to Schooler Kane
criteria. The average CPZ equivalents among study
subjects who satisfied the Schooler Kane criteria
was 345.83 (±101.73) and the
average CPZ equivalents among study subjects who
did not satisfy the Schooler Kane criteria was
242.51 (± 96.88). The difference was statistically
significant (p<.001).
|
Fig.
4: Comparison of duration of
Chlorpromazine equivalents according to
the Schooler Kane criteria
|
Discussion:
Tardive dyskinesia
is a movement disorder which can occur due to the
use of antipsychotics. The oral, buccal, and
lingual regions of the face are often afflicted by
TD; while the trunk and limbs may also be
affected, they are typically less severely
affected. Athetoid or choreiform movements are
common terms used to characterise involuntary
movements. (8)
Women are more
likely than men to develop tardive dyskinesia,
particularly in patients who are middle-aged to
elderly. Patients who are elderly may be more
susceptible to tardive dyskinesia because of
age-related changes in the body and brain. (12)
Tardive dyskinesia
(TD) gets its name from the slow or tardive onset
of involuntary movements of the face, lips,
tongue, trunk, and extremities. A number of
theories have been put forth regarding the
mechanism, including the following: oxidative
stress, gamma-aminobutyric acid (GABA) depletion,
cholinergic deficiency, prolonged blockade of
postsynaptic dopamine receptors resulting in
dopamine receptor hypersensitivity; altered
synaptic plasticity; neurotoxicity; and impaired
neuroadaptive signalling. (13)
In this study, the
majority of patients were in the 51–60 age range,
with an average age of 45.39 years (SD=12.6336)
(Table 1). This almost exactly matches the
findings of the study by Sahel Hemmati et al (14),
which revealed that the study's youngest and
oldest participants were, respectively, 19 and 60
years old. Half of the sample group had an average
age of 44 or less, according to the average, mean,
and standard deviation. This is consistent with
the research done by Daniel SJ et al (15), where
the sample's average age was 43.75 years (SD
10.5). This is consistent with the study by Bhatia
T et al (16), in which the mean age was 47.6±11.09
years.
Out of 207
participants in this study, 139 (67.1%) had
schizophrenia or an unexplained psychosis, and 68
(32.9%) had bipolar (Figure 1). This is consistent
with a research by Abdeta T et al (17), in which a
maximum of 79.5% of participants were diagnosed
with schizophrenia. This is almost in line with a
research by N Gatere et al(18), in which 48 people
(28.8%) had bipolar and 91 people (45%) had
schizophrenia or an unexplained psychosis. This
nearly matches the findings of the study by Bakker
PR et al,(19) which found that schizophrenia and
psychosis, as defined by DSM-IV Axis I, accounted
for 69.6% and 5.3%, respectively, of diagnoses.
Out of 207 study
participants, 116 utilized the drug Risperidone in
this study (Table 2). This almost matches the
findings of Bhatia T's(16) study. Among 96
patients with TD, Risperidone was the only
antipsychotic medication used in 39.6% of cases
and in 47.9% of cases. This is consistent with
research by Achalia RM et al(11), in which
Risperidone was the most often prescribed
antipsychotic (n = 50) for 90 individuals using
SGAs. This is consistent with the Santhanakrishna
et al study(20), in which 45.71% of patients
received Risperidone as their most frequent
prescription.
According to Bhatia
T et al,(16) atypical antipsychotic medications
are known to elicit extra- pyramidal symptoms less
frequently (only 1% of the time) and to be related
with milder TD than older, traditional
antipsychotic medications (5%). In a high-risk
group of elderly patients, Jeste DV et al(21) came
to the conclusion that Risperidone was
considerably less likely to cause TD than the
traditional neuroleptic medicines, at least over a
nine-month period.
A maximum of 32
study participants received treatment for 5 years
in this trial (Figure 2). According to a study by
Abdeta T et al,(17) 49% of participants took their
prescribed medications for more than five years.
This is consistent with the study of Assefa Kumsa
et al(22), in which 246 (60.0%) of the patients
had received treatment for an average of 4.8
years, with an SD of 3.9 years, ranging from 1 to
5 years.
Of the 207
participants in this study, 99 are urban dwellers,
and 111 are rural (Table 1).
This
contradicts research by Assefa Kumsa et al
(22), in which 206 study participants lived in
urban regions and 204 in rural areas. Out of 207
study participants, 71.5% were illiterate in this
study (Table 1). This contradicts a research by
Abdeta T et al (17), in which 42.4% of respondents
had educational status in grades 1-6.
In this study, 79
study participants received a cumulative
equivalent dose of 200 mg of Chlorpromazine (Table
5). According to a study by Abdeta T et al (17),
74.2% of the study individuals had a cumulative
equivalent dose of 100–<400 mg of
Chlorpromazine. This is almost in line with the
findings of the study by N Gatere et al (18), in
which 133 of the study individuals received less
than 500 mg of Chlorpromazine cumulative
equivalent dose each day. The average
Chlorpromazine-equivalent dose used in this
population in the past was 336.95, according to a
study by Woniak K et al.(23)
Hyperkinetic
choreiform involuntary motions that frequently
change in severity are referred to as dyskinesia.
TD was evaluated using the Abnormal Involuntary
Movement Scale (AIMS)(9) and the Schooler and Kane
criteria(10) were used to define the case. These
criteria required (i) the presence of moderate
dyskinesia in at least one body area or mild
dyskinesia in at least two body parts, and (ii)
the absence of other conditions that cause
abnormal involuntary movements.
Of the 207
participants in this study 18 (8.7%) had mild
Tardive Dyskinesia, and 30 (14.5%) had significant
Tardive Dyskinesia (Figure 3). Tardive Dyskinesia
affected 23.2% of people overall, according to
AIMS (facial and oral) score. This is consistent
with a study by Bakker PR et al,(19) which found
that the overall prevalence of tardive dyskinesia
for orofacial TD ranged from 21.7 to 32.5%.
According to the study done by O Gureje et al,(24)
the prevalence of orofacial involuntary movements
was 26% and was determined by an AIMS rating of 2
on any one orofacial area. According to the
research done by Anusa A,(25) mild Tardive
Dyskinesia affected 24 people (21.1%), whereas
moderate Tardive Dyskinesia affected 28 people
(24.5%). According to AIMS (extremity) score, none
of the study participants had TD of extremities.
This is approximately in line with the research
done by Anusa A et al, (25) where the maximum
percentage of subjects that met the study's goals
(extremity) was 68.4%.
According to AIMS –
trunk scores, none of the study participants had
truncal TD. This is almost in line with a study by
Bakker PR, (19) where the majority of individuals
were normal for limb truncal TD goals
(extremity).The average AIMS total score,
according to Bhatia T et al,(16) was 2.29±3.25
(6.29±3.48 for TD positive cases), whereas the
average orofacial score, average extremities
score, and average trunk score were each 0.53±1.29
(1.72±1.90 for TD positive cases) and 0.09±0.29
(0.32±0.47 for TD positive).
This is in contrast
to a study by Santhanakrishna et al (20), which
found that among extrapyramidal symptoms,
involvement of the extremities was most common in
42.5% of cases, followed by involvement of the
trunk in 35.7% of cases and of the face and mouth
in 21.42% of cases. In 45% of the patients, the
severity of the extrapyramidal symptoms was
moderate, while in 25.71% of the patients, it was
mild.
This study shows how
the study volunteers were distributed based on the
overall study goal score. 13 (6.3%) and 20 (9.7%)
of the 207 study participants had mild and
significant Tardive Dyskinesia, respectively
(Table 4). According to AIMS (global), 16% of
people worldwide had Tardive Dyskinesia. This is
in line with the research done by Abdeta T et al
(17), who found that 14.6% of participants in the
current study had TD, with a range of 10.76% to
18.4%. This almost matches the findings of the
study by Taye H et al.,(26) which found that 11.9%
of patients experienced TD and that the prevalence
of TD among psychiatric patients taking
first-generation antipsychotics is similar to
that. This is consistent with the research done by
Achalia RM et al.,(11) which found that 26.4% of
the sample overall had probable TD.
This study shows how
the study subjects were distributed using the
Schooler-Kane criteria (Table 4). 31 (14.5%) of
the 207 participants in the research exhibited
mild Tardive Dyskinesia. According to Schooler
Kane criterion, Tardive Dyskinesia by Woerner MG
et al(4), was present in 14.5% of people
nationwide. According to a research by Gatere et
al(18), 12% of participants had TD (as defined by
the Schooler Kane criteria). This is in contrast
to a research by Munshi T et al(27), where 29% of
participants met the Schooler Kane criteria for
TD.
In this study,
Chlorpromazine equivalents are compared using
Schooler-Kane criteria (Figure 4). According to
study subjects who met the Schooler Kane
requirements, the average Chlorpromazine
equivalents were 345.83 (±101.73), whereas those
who did not met the criterion had Chlorpromazine
equivalents of 242.51 (±96.88). There was a
statistically significant difference (p<.001).
This is consistent with the research done by
Wozniak K et al(23) in which the average
chlorpromazine-equivalent dose used in this
population in the past who met the Schooler Kane
requirements were 336.95.
Future studies could
include a bigger sample size. Studies could also
be done with patients who are followed up
longitudinally.
Strengths:
The strengths of
this study are that standard diagnostic criteria
and rating scales were used. A good sample size
was taken, involving both males and females. Also,
patients with chronic mental illness from two
rehabilitation centres were considered. A single
examiner has conducted the examination process,
eliminating inter-observer variability.
Limitations:
The limitation of
this study is that it is necessary to conduct
studies with sizable samples that are tracked over
long periods of time to confirm the results of the
current study.
Conclusion:
Many patients with
psychotic diseases experienced movement
difficulties brought on by conventional
antipsychotics, which were viewed as burdensome
and stigmatizing events. It was also discovered
that chlorpromazine comparable dosages were
significantly higher among participants with TD.
Orofacial TD had the highest overall prevalence.
Designing treatment guidelines, expanding the
availability of medications with minimum adverse
effects, and providing psychoeducation on related
aspects are crucial.
Funding: Nil
Conflict of Interest: Nil
Disclosure:
This paper has been presented at
KANCIPS and IPSOCON conferences 2023. It has
received the HS Subramanyam award for the best PG
oral paper.
References:
- American Psychiatric Association. Diagnostic
and Statistical Manual of Mental Disorders
(DSM-5-TR)DSM. Available from: https://www.psychiatry.org:443/psychiatrists/practice/dsm
- Glazer WM. Review of incidence studies of
tardive dyskinesia associated with typical
antipsychotics. J Clin Psychiatry.
2000;61 Suppl 4:15–20.
- Tarsy D, Baldessarini RJ. Epidemiology of
tardive dyskinesia: is risk declining with
modern antipsychotics? Mov Disord Off J Mov
Disord Soc. 2006 May;21(5):589–98.
- Woerner MG, Alvir JM, Saltz BL, Lieberman JA,
Kane JM. Prospective study of tardive dyskinesia
in the elderly: rates and risk factors. Am J
Psychiatry. 1998 Nov;155(11):1521–8.
- Jeste DV, Caligiuri MP, Paulsen JS, Heaton RK,
Lacro JP, Harris MJ, Bailey A, Fell RL, McAdams
LA. Risk of tardive dyskinesia in older
patients. A prospective longitudinal study of
266 outpatients. Arch Gen Psychiatry.
1995 Sep;52(9):756-65. Available from: https://pubmed.ncbi.nlm.nih.gov/7654127/
- Correll CU, Schenk EM. Tardive dyskinesia and
new antipsychotics. Curr Opin Psychiatry. 2008
Mar;21(2):151–6.
- Woerner MG, Correll CU, Alvir JMJ, Greenwald
B, Delman H, Kane JM. Incidence of tardive
dyskinesia with risperidone or olanzapine in the
elderly: results from a 2-year, prospective
study in antipsychotic-naïve patients. Neuropsychopharmacol
Off Publ Am Coll Neuropsychopharmacol. 2011
Jul;36(8):1738–46.
- Mori Y, Takeuchi H, Tsutsumi Y. Current
perspectives on the epidemiology and burden of
tardive dyskinesia: a focused review of the
clinical situation in Japan. Ther Adv
Psychopharmacol. 2022 Dec
26;12:20451253221139608.
- Guy W. ECDEU assessment manual for
psychopharmacology. Rev. 1976. Rockville, Md:
U.S. Dept. of Health, Education, and Welfare,
Public Health Service, Alcohol, Drug Abuse, and
Mental Health Administration, National Institute
of Mental Health, Psychopharmacology Research
Branch, Division of Extramural Research
Programs; 1976. 603 p.
- Schooler NR, Kane JM. Research diagnoses for
tardive dyskinesia. Arch Gen Psychiatry. 1982
Apr;39(4):486–7.
- Achalia RM, Chaturvedi SK, Desai G, Rao GN,
Prakash O. Prevalence and risk factors
associated with tardive dyskinesia among Indian
patients with schizophrenia. Asian J
Psychiatry. 2014 Jun;9:31–5.
- Vasan S, Padhy RK. Tardive Dyskinesia. In:
StatPearls. Treasure Island (FL): StatPearls
Publishing; 2023. Available from:
http://www.ncbi.nlm.nih.gov/books/NBK448207/
- Cornett EM, Novitch M, Kaye AD, Kata V, Kaye
AM. Medication-Induced Tardive Dyskinesia: A
Review and Update. Ochsner J.
2017;17(2):162–74.
- Hemmati S, Astaneh AN, Solemani F, Vameghi R,
Sajedi F, Tabibi N. A survey of the tardive
dyskinesia induced by antipsychotic drugs in
patients with schizophrenia. Iran J
Psychiatry. 2010;5(4):159–63.
- Daniel SJ, Kannan PP, Malaiappan M, Anandan H.
Relationship between Awareness of Tardive
Dyskinesia and Awareness of Illness in
Schizophrenia. Int J Sci Stud.
2016;4(7):17-20
- Bhatia T, Sabeeha MR, Shriharsh V, Garg K,
Segman RH, Uriel HL, et al. Clinical and
familial correlates of tardive dyskinesia in
India and Israel. J Postgrad Med. 2004;50(3):167–72;
discussion 172.
- Abdeta T, Tolessa D, Tsega W. Prevalence and
Associated Factors of Tardive Dyskinesia Among
Psychiatric Patients on First-Generation
Antipsychotics at Jimma University Specialized
Hospital, Psychiatric Clinic, Ethiopia:
Institution Based on A Cross-Sectional Study. Journal
of Psychiatry and Psychiatric Disorders
2019;3: 179-190. Available from: http://www.fortunejournals.com/articles/prevalence-and-associated-factors-of-tardive-dyskinesia-among-psychiatric-patients-on-firstgeneration-antipsychotics-at-jimma-univ.html
- Gatere N, Othieno CJ, Kathuku DM. Prevalence
of tardive dyskinesia among psychiatric
in-patients at Mathari Hospital, Nairobi.
East Afr Med J. 2002 Oct;79(10):547–9.
- Bakker PR, de Groot IW, van Os J, van Harten
PN. Long-stay psychiatric patients: a
prospective study revealing persistent
antipsychotic-induced movement disorder. PloS
One. 2011;6(10):e25588.
- Santhanakrishna KR, Revanakar S,
Srirangapattna C. Prevalence of Extrapyramidal
Side Effects in Patients on Antipsychotics Drugs
at a Tertiary Care Center5. J Psychiatry.
2017;20(5). Available from: https://www.omicsonline.org/open-access/prevalence-of-extrapyramidal-side-effects-in-patients-on-antipsychotics-drugsat-a-tertiary-care-center5-2378-5756-1000419.php?aid=92481
- Jeste DV, Lacro JP, Bailey A, Rockwell E,
Harris MJ, Caligiuri MP. Lower incidence of
tardive dyskinesia with risperidone compared
with haloperidol in older patients. J Am
Geriatr Soc. 1999 Jun;47(6):716–9.
- Kumsa A, Girma S, Alemu B, Agenagnew L.
Psychotropic Medications-Induced Tardive
Dyskinesia and Associated Factors Among Patients
with Mental Illness in Ethiopia. Clin
Pharmacol. 2020 Dec 1;12:179-187. doi:
10.2147/CPAA.S285585. Available from: https://pubmed.ncbi.nlm.nih.gov/33293875/
- Woźniak K, Kłoszewska I. Clinical assessment
of antipsychotic-induced extrapyramidal symptoms
in nursing home residents with schizophrenia. Psychiatr
Psychol Klin. 2016 Mar 31;16(1):7–14.
- Gureje O. Topographic subtypes of tardive
dyskinesia in schizophrenic patients aged less
than 60 years: relationship to demographic,
clinical, treatment, and neuropsychological
variables. J Neurol Neurosurg Psychiatry. 1988
Dec;51(12):1525–30.
- Anusa AM, Thavarajah R, Nayak D, Joshua E, Rao
UK, Ranganathan K. A Study on Drug-Induced
Tardive Dyskinesia: Orofacial Musculature
Involvement and Patient’s Awareness. J
Orofac Sci. 2018;10(2):86–95.
- Taye H, Ebrahim J. Antipsychotic Medication
Induced Movement Disorders: The Case of Amanuel
Specialized Mental Hospital, Addis Ababa,
Ethiopia. Am J Psychiatry Neurosci. 2014
Jan 1;2:76.
- Adam UU, Husain N, Haddad PM, Munshi T, Tariq
F, Naeem F, et al. Tardive dyskinesia in a South
Asian population with first episode psychosis
treated with antipsychotics. Neuropsychiatr
Dis Treat. 2014 Oct 14;10:1953–9.
|



















