|
Introduction
Enterococci
are one of the important cause of infections,
especially in the hospitalised patients. They are
normal commensals found in the intestines of the
humans. They are intrinsically resistant to
majority of the commonly used antibiotics in the
hospital like cephalosporins, aminoglycosides,
clindamycin etc. Therefore, these bacteria can
successfully cause nosocomial infections. (1)
There are various
known species of enterococci which can cause
infections in humans. E. faecium
infections are generally found to be more
difficult to treat compared to other species of
enterococci. In this study, we determined the
proportion of the enterococcal infections due to
the E. faecium species and the
differences in the epidemiology and susceptibility
profiles of the E. faecium isolates
compared to E. faecalis isolates in a
tertiary care hospital in Mangalore, in southern
India.
Methods
This prospective
cohort study was conducted at Father Muller
Medical College Hospital, Mangalore for a period
of six months from June to November 2023, after
ethical clearance from the Father Muller Institute
Ethics Committee (FMIEC/CCM /297 /2023).
Inclusion
criteria: Non repetitive clinical isolates
of Enterococcus species from various
clinical specimens like urine, exudates and blood
received in the microbiology laboratory.
Exclusion
criteria: The isolates of enterococci from
the stool and respiratory sample were excluded
from the study.
Procedure:
The samples were
cultured using 5% sheep blood agar (HiMedia
Laboratories Pvt. Ltd., Mumbai, India) and
MacConkey agar (HiMedia Laboratories Pvt. Ltd.,
Mumbai, India Bac T/Alert aerobic culture bottle
(bio Mérieux, France) was used for blood culture.
All the suspected enterococcal isolates were
identified and speciated by standard biochemical
tests. The small translucent colonies on the 5%
sheep blood agar and corresponding pinpoint
magenta pink colonies were selected for colony
smear and Gram staining. The isolates were tested
for production of catalase enzyme. The
Gram-positive cocci in pairs, which did not
produce catalase enzyme, were further tested for
hydrolysis of bile esculin, fermentation of
mannitol, arabinose and motility. The isolates
which hydrolysed bile esculin were identified as Enterococcus
species. The non motile isolates were speciated as
E. faecalis or E. faecium
based on fermentation of arabinose. The
confirmation of identification of enterococci was
done by Bruker Daltonics Microflex LT/SH MALDI-MS
System (Bruker Daltonics, Germany).
Antibiotic
susceptibility testing was done by modified Kirby
Bauer disc diffusion method according to Clinical
Laboratory Standards Institute (CLSI) guidelines
(3). Antibiotic discs from HiMedia Laboratories
Pvt. Ltd., Mumbai, India were used. Enterococcus
faecalis ATCC strain 29212, Staphylococcus
aureus ATCC 25923 and Escherichia coli
ATCC 25922 were used as quality controls for
antibiotics. Ampicillin 10µg, high level
gentamicin 120 µg, nitrofurantoin 300 µg,
ciprofloxacin 5 µg, levofloxacin 5 µg, vancomycin
30 µg, teicoplanin 30 µg and linezolid 30 µg discs
were used for antibiotic susceptibility testing.
Statistical
Analysis:
The statistical
analysis was done using SPSS version 23, IBM, USA.
The categorical variables have been expressed in
terms of percentages and frequencies. The
continuous variables are expressed as mean or
median based on the type of distribution. The chi
square test or Fishers exact test has been used as
test of significance between the categorical
variables. Independent t test or Man Whitney U
test were used for the analysis of continuous
variables.
Results
In the present
study, 132 isolates of the enterococci from the
urine, exudates and blood were included. The
median age of the patients with the enterococci in
clinical samples was 59 years.
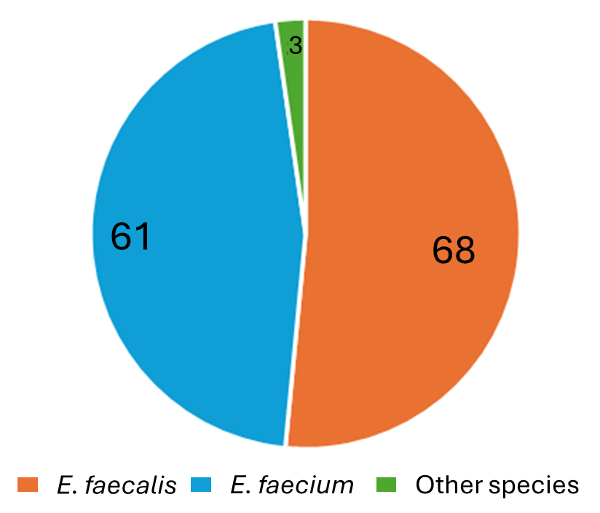
Among the isolates
included in the study, sixty-six (50%) each were
from males and females. On speciation, 68/132
(51.51%) isolates were identified as E.
faecalis, 61/132 (46.21%) as E.
faecium and2/132 (1.53%) isolates as E.
gallinarum and 1/132 (0.75%) as E.
raffinosus.
|
| Fig
1: Species distribution of the
enterococcal isolates |
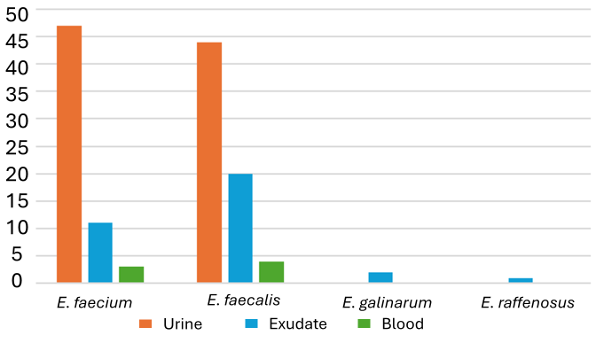
|
| Fig 2: Sample wise
distribution of the various enterococcal
species. |
Ninety-one (68.9%)
of the enterococcal isolates were from urine
samples, 34 (25.8%) of the isolates were from
exudates and 7 (5.3%) of the isolates were from
blood culture samples as shown in Fig 2.
Ninety-six (72.72%)
of the enterococcal isolates were from patients
admitted to the hospital, whereas 36 (27.27%) of
isolates were from the outpatient department. The
overall susceptibility testing of all the
enterococcal isolates is as in the Table 1. The
highest rate of resistance was against
fluoroquinolones, high level gentamicin,
ampicillin, nitrofurantoin, vancomycin and
teicoplanin. No resistance was detected in our
isolates against linezolid.
|
Table 1: Susceptibility of the
enterococcal isolates
|
|
Antibiotic Susceptibility Agents
|
Sensitive strains
|
Resistant strains
|
|
Number(n)
|
Percentage (%)
|
Number(n)
|
Percentage (%)
|
|
Ampicillin
|
86
|
65.15
|
46
|
34.85
|
|
High level Gentamicin
|
72
|
54.55
|
60
|
45.45
|
|
Ciprofloxacin
|
58
|
43.94
|
74
|
56.06
|
|
Levofloxacin
|
59
|
44.70
|
73
|
55.30
|
|
Nitrofurantoin
|
102
|
77.27
|
30
|
22.73
|
|
Vancomycin
|
124
|
93.93
|
10
|
7.58
|
|
Teicoplanin
|
126
|
95.45
|
06
|
4.55
|
|
Linezolid
|
132
|
100
|
00
|
00
|
The characteristics
of E. faecium compared with E.
faecalis are shown in Table 2. On
statistical analysis, it was found that E.
faecium was more significantly isolated
from the urine samples, whereas E. faecalis
was significantly more in the exudates. Also, E.
faecium was isolated significantly more in
the samples from the inpatient department. E.
faecium was found to be significantly more
resistant to ampicillin, high level gentamicin,
nitrofurantoin and vancomycin.
|
Table 2: Characteristics of the E.
faecium isolates
|
|
Enterococcus faecium
|
Enterococcus faecalis
|
P value
|
|
Median age
|
59
|
60
|
0.732*
|
|
Male
|
32 (52.5%)
|
33 (48.5%)
|
0.656†
|
|
Female
|
29 (47.5%)
|
35 (51.5%)
|
|
Urine isolates
|
47 (77%)
|
41 (60.3%)
|
0.041†
|
|
Exudate isolates
|
11 (18%)
|
23 (33.8%)
|
0.042†
|
|
Blood isolates
|
3 (4.9%)
|
4 (5.9%)
|
1.000‡
|
|
Isolates from inpatient departments
|
50 (82.0%)
|
43 (63.2%)
|
0.018†
|
|
Outpatient
|
11 (18.0%)
|
25 (36.8%)
|
|
Antibiotic Susceptibility
|
|
Ampicillin
|
Susceptible
|
26 (42.6%)
|
58 (85.3%)
|
0.000†
|
|
Resistant
|
35 (57.4%)
|
10 (14.7%)
|
|
High level Gentamicin
|
Susceptible
|
28 (45.9%)
|
43 (63.2%)
|
0.048†
|
|
Resistant
|
33 (54.1%)
|
25 (36.8%)
|
|
Ciprofloxacin
|
Susceptible
|
22 (36.1%)
|
36 (52.9%)
|
0.054†
|
|
Resistant
|
39 (63.9%)
|
32 (47.1%)
|
|
Levofloxacin
|
Susceptible
|
23 (37.7%)
|
36 (52.9%)
|
0.083†
|
|
Resistant
|
38 (62.3%)
|
32 (47.1%)
|
|
Nitrofurantoin
|
Susceptible
|
38 (62.3%)
|
62 (91.2%)
|
0.000†
|
|
Resistant
|
23 (37.7%)
|
6 (8.8%)
|
|
Vancomycin
|
Susceptible
|
54 (88.5%)
|
67 (98.5%)
|
0.026‡
|
|
Resistant
|
7 (11.5%)
|
1 (1.5%)
|
|
Teicoplanin
|
Susceptible
|
56 (91.8%)
|
67 (98.5%)
|
0.100‡
|
|
Resistant
|
5 (8.2%)
|
1 (1.5%)
|
|
Linezolid
|
Susceptible
|
61 (100%)
|
68 (100%)
|
-
|
|
Resistant
|
-
|
-
|
|
* - Mann Whitney U test; †- Chi square
test; ‡- Fishers exact test
|
Discussion
Enterococci are
important cause of infections in a wide range of
patients. They cause urinary tract infections,
blood stream infections and skin and soft tissue
infections. These infections tend to be resistant
to some of the common antibiotics used in the
hospitals. E. faecalis and E.
faecium are the 2 common species isolated
from the clinical samples.
The enterococcal
infections occurred in all the age groups of
patients in the hospital. The median age of the
patients with enterococcal isolates was 59 years.
This is in contrast to studies by Yadav and
Agarwal, and Mussadiq et al, where the maximum
number of isolates from age group 20-29 years and
21-40 years respectively. (4,5) The isolates were
equally distributed among males and females. In
contrast, Yadav and Agarwal observed that the
enterococci were isolated more among the female
patients. (4)
On speciation, 68
(51.51%) isolates were identified as Enterococcus
faecalis, 61 (46.21%) as Enterococcus
faecium and 2 (1.51%) isolates as Enterococcus
gallinarum and 1 (0.75%) as Enterococcus
raffinosus. Most of the studies in the
different places of India show a lower proportion
of E. faecium. The proportion of the E.
faecium in the different studies in New
Delhi, West Bengal, Sikkim have reported a
proportion of E. faecium varying between
8.5% to 28.8%. (6–11) Similar reports are also
available worldwide. (12,13) On the contrary,
Yadav and Agarwal, in a study conducted at Lucknow
reported higher proportion (47.5%) of E.
faecium. (4) Interestingly, two studies
from Mangalore show very different proportions of
E. faecium. (14,15) This shows there is
a varying proportion of E. faecium
infection in the country and the city, at
different time periods. The different studies have
reported few uncommon species of enterococci like
E. hirae, E. durans, E. avium, E. mundtii, E.
gallinarum etc. (6,7,9,10) This is similar
to our study where E. gallinarum and
E. raffinosus were detected. Hence it is
seen that majority of the enterococcal infections
in the country are caused by E. faecalis and
E. faecium.
Ninety-one (68.9%)
of the enterococcal isolates were from urine
samples, 34 (25.8%) of the isolates were from
exudates and 7 (5.3%) of the isolates were from
blood culture samples. This in contrast to the
various studies in India, where about 80% of the
isolates of enterococcal isolates were from the
urine samples. (4,6) A higher proportion of the
enterococci were reported from the exudate samples
in our facility.
Highest number of
the isolates in our study were resistant to
fluoroquinolones (55% and 56%). This is similar to
high resistance to fluoroquinolones, reported in
the various studies in the country. (4,7) Most of
the studies in the country report resistance of
enterococci to high level gentamicin of 30%- 55%.
(4,7,10,11,16) High level gentamicin resistance
was detected in 45.45% of the enterococcal
isolates in the present study. Ampicillin is a
good antibiotic to treat the enterococcal
infections, if found to be susceptible. Resistance
to ampicillin was detected in 34.85% of the
enterococcal isolates. Similar resistance rate to
ampicillin was documented by few studies, (6)
whereas other studies showed higher resistance
rates. (4,7) Nitrofurantoin is a very good drug
for susceptible enterococcal urinary tract
infections. Nitrofurantoin resistance was detected
in 22.73% of the isolates which is concordant with
other studies in the country. (6,7)
With the increased
use of vancomycin and teicoplanin in the hospital,
there has been development of enterococcal
vancomycin resistance. Vancomycin resistance was
observed in 7.58% of the isolates and teicoplanin
resistance in 4.55% of the isolates. This is in
contrast to a meta-analysis, where pooled
vancomycin resistance among the enterococcal
isolates from India was 12.4%. (17) There are
studies with various different rates of vancomycin
resistance in the country. (4,7–11) Chakraborty et
al, in a study in West Bengal in the year 2015,(9)
reported no vancomycin resistance, whereas Meena
et al, in a study published in 2017, reported a
high 37% of vancomycin resistant enterococci in
New Delhi. (8) The teicoplanin resistance in the
present study is lesser than in other similar
studies. (7,16)
There was no
linezolid resistance among all the enterococcal
isolates in the present study compared to a study
at New Delhi, where in a span of 3 years, 202
linezolid resistant enterococcal strains were
isolated. (18) Various studies in the country have
reported various rates of linezolid resistance.
(4,7,10)
The isolates of E.
faecium did not differ from other isolates
in terms of median age of the patients. The median
age of the patients in both the groups was 59
years. There was no significant difference in
terms of the gender of the patients. The E.
faecium, were more frequently isolated
from urine samples (77% vs 60.3 % p value = 0.041)
and less from the exudate samples (18% vs 33.8%, p
value = 0.042), when compared to E. faecalis.
There was no significant difference among the E.
faecium isolates in terms of the blood
sample (4.9% vs 5.9%, p value = 1.000). It was
observed in the study that E. faecium isolates
were isolated significantly more from the samples
of the inpatient departments when compared to E.
faecalis isolates (82% vs 63.2% p value =
0.018)
It was observed that
E. faecium isolates were significantly
more resistant to ampicillin (57.4% vs 14.7% p
value = 0.000). In their study at Etawah, Uttar
Pradesh, Musaddiq et al reported much higher
resistance to ampicillin in both E. fecium
and E. fecalis isolates (90.32% vs 84.78%)
(5). The E. faecium isolates were also
significantly more resistant to the high-level
gentamicin (54.1% vs 36.8% p value- 0.048),
nitrofurantoin (37.7% vs 8.8%, p value =0.000),
than E. faecalis isolates. E.
faecium isolates were found to be more
resistant, also to ciprofloxacin, levofloxacin,
teicoplanin (8.2% vs 1.5%, p value = 0.100),
albeit not significantly when compared to the E.
faecalis isolates. Similarly, Das et al,
documented higher resistance rates of E.
faecium, compared to E. faecalis
isolates at New Delhi, (7) Similar higher
difference in resistance rates was observed in a
study in Tabriz, Iran. (12)
The E. faecium
isolates were significantly more resistant to
vancomycin than E. faecalis (11.5% vs
1.5%, p value = 0.026) which is concordant with
other studies. (4,7,12,16,19)
Limitations of the
study is that it is a single centre study and the
only the phenotypic characterisation of the
enterococcal isolates was done. This study
demonstrates a higher proportion of E.
faecium causing clinical infections than in
other parts of the country. E. faecium
in our study has significantly higher resistance
rates to ampicillin, high level gentamicin,
nitrofurantoin and vancomycin. Factors leading to
increased rates of E. faecium infections
and its antibiotic resistance need to be looked
for and addressed promptly.
Conclusion
In this study we
found that the proportion of the E. faecium
among the clinical isolates is higher compared to
many other studies. This is of concern, as the
resistance rates to various antibiotics are higher
among the E. faecium. The E.
faecium isolates were mostly from
inpatients indicating antibiotic overuse may be a
causal factor. There were significantly higher
rates of resistance seen among the E. faecium
isolates compared to E. faecalis
isolates in our study. Fortunately, the overall
rate of resistance to vancomycin is low and none
of the isolates were resistant to linezolid.
Strict infection control and antimicrobial
stewardship measures need to be taken in the
hospitals to decrease the prevalence of the E.
faecium infections and the resistance among
the enterococci to prevent any adverse outcome.
References
- Farsi S, Salama I, Escalante-Alderete E,
Cervantes J. Multidrug-Resistant Enterococcal
Infection in Surgical Patients, What Surgeons
Need to Know. Microorganisms.
2023;11(2):238..
- Procop GW. Koneman’s Color Atlas and Textbook
of Diagnostic Microbiology. Wolters Kluwer
Health; 2017.
- CLSI. Performance Standards for Antimicrobial
Susceptibility Testing. 33rd ed.
- Yadav R, Agarwal L. Enterococcal infections in
a tertiary care hospital, North India. Ann
Afr Med. 2022;21(3):193.
- Mussadiq S, Verma RK, Singh DP, et al.
Vancomycin Resistant Enterococcal Urinary Tract
Infection: A Potential Threat. Nat J Lab
Med. 2023;12(2):MO01-5.
- Mohanty S, Behera B. Antibiogram Pattern and
Virulence Trait Characterization of Enterococcus
Species Clinical Isolates in Eastern India: A
Recent Analysis. J Lab Physicians.
2022;14(03):237–46.
- Das A, Dudeja M, Kohli S, et al. Genotypic
characterization of vancomycin-resistant
Enterococcus causing urinary tract infection in
northern India. Indian J Med Res.
2022;155(3):423.
- Meena S, Mohapatra S, Sood S, et al.
Revisiting Nitrofurantoin for Vancomycin
Resistant Enterococci. J Clin Diagn Res. 2017;11(6):DC19-22.
- Chakraborty A, Pal N, Sarkar S, et al.
Antibiotic resistance pattern of Enterococci
isolates from nosocomial infections in a
tertiary care hospital in Eastern India. J
Nat Sci Biol Med. 2015;6(2):394.
- Yadav G, Thakuria B, Madan M, et al. Linezolid
and Vancomycin Resistant Enterococci: A
Therapeutic Problem. J Clin Diagn Res.
2017;11(8):GC07-11.
- Sachan S, Rawat V, Umesh, et al.
Susceptibility pattern of enterococci at
tertiary care hospital. J Glob Infect Dis.
2017;9(2):73.
- Jahansepas A, Aghazadeh M, Rezaee MA, et al.
Occurrence of Enterococcus faecalis
and Enterococcus faecium in Various
Clinical Infections: Detection of Their Drug
Resistance and Virulence Determinants. Microb
Drug Resist. 2018 Jan;24(1):76–82.
- Álvarez-Artero E, Campo-Nuñez A, García-García
I, et al. Urinary tract infection caused by Enterococcus
spp.: Risk factors and mortality. An
observational study. Rev Clínica Esp Engl Ed.
2021;221(7):375–83.
- Bhat NR, Shivashankar SBK, Dhanashree B.
Antibiogram of Urinary Enterococcus Isolates
from a Tertiary Care Hospital. Infect Disord
- Drug Targets. 2021;21(1):146–50.
- Shridhar S, Dhanashree B. Antibiotic
Susceptibility Pattern and Biofilm Formation in
Clinical Isolates of Enterococcus spp.
Interdiscip Perspect Infect Dis. 2019
3;2019:1–6.
- Yangzom T, Kumar Singh TS. Study of vancomycin
and high-level aminoglycoside-resistant
Enterococcus species and evaluation of a rapid
spot test for enterococci from Central Referral
Hospital, Sikkim, India. J Lab Physicians.
2019 ;11(03):192–9.
- Smout E, Palanisamy N, Valappil SP. Prevalence
of vancomycin-resistant Enterococci in India
between 2000 and 2022: a systematic review and
meta-analysis. Antimicrob Resist Infect
Control. 2023 21;12(1):79.
- Rani V, Aye NK, Saksena R, et al. Risk factors
and outcome associated with the acquisition of
MDR linezolid-resistant Enterococcus faecium: a
report from tertiary care centre. Eur J Clin
Microbiol Infect Dis Off Publ Eur Soc Clin
Microbiol. 2024;43(4):767–75.
- Kilbas I, Ciftci IH. Antimicrobial resistance
of Enterococcus isolates in Turkey: A
meta-analysis of current studies. J Glob
Antimicrob Resist. 2018;12:26–30.
|



















