|
Introduction
In
hospitalized patients, especially in critical care
patients, candidemia accounts for considerable
morbidity and mortality. It is the most serious
systemic infection produced by Candida
with a mortality rate of 20-40%, seen frequently
in patients with risk factors such as prolonged
antibiotic therapy, invasive surgery, indwelling
intravenous catheters, prosthetic devices,
hyperalimentation fluids, chemotherapy. (1,2)
Majority of
candidemia episodes are caused by Candida
albicans, Candida glabrata, Candida
parapsilosis, Candida tropicalis and Candida
krusei. A change in the epidemiology of candida
infections, characterized by a progressive shift
from a predominance of C. albicans to
non-albicans Candida species such as C.
tropicalis, C. glabrata, and C.
krusei has been reported from many
countries around the world [3-6]. Among these, C.
auris is a newly noticed,
multidrug-resistant yeast leading to outbreaks in
various geographical locations. (7) This changing
scenario is concerning as these species have
reduced susceptibilities to antifungal agents. It
is observed that C. albicans is usually
susceptible to fluconazole, but there is a rise in
primary resistance to fluconazole in C.
glabrata, C. tropicalis, and C. parapsilosis.
(2,6) The situation is more complicated with the
arrival of C. auris which is associated
with health care infections with high rates of
clinical treatment failure. (7)
Knowledge of local
species epidemiology and antifungal susceptibility
is important when considering therapy. This study
was performed to speciate bloodstream Candida
isolates by employing MALDI-TOF analysis and to
assess their risk factors in patients admitted in
our tertiary care hospital.
Materials and Methods
This ambispective
study of 2 years' duration was performed from
April 2022 to March 2024 with Institutional Ethics
Committee approval (FMIEC/CCM/550/2023). Data was
retrieved from the laboratory information system.
All the blood cultures received in the
microbiology laboratory were included in the
study. Any repeat isolate from the same patient
was excluded from the study.
Blood cultures were
processed using Bac T/Alert aerobic culture bottle
(bio Mérieux, France) and were incubated for 5
days at 37°C. Blood cultures growing yeast were
subjected to Gram stain and sub-cultured on blood
agar (HiMedia Laboratories Pvt. Ltd., Mumbai,
India). and Sabouraud’s dextrose agar (SDA)
(HiMedia Laboratories Pvt. Ltd., Mumbai, India).
Species level identification of Candida
was performed by using Bruker Daltonics Microflex
LT/SH MALDI-MS System (Bruker Daltonics, Germany)
with maldi-Biotyper software and MBT Compass data
V12.0.0.0_10833. MALDI-TOF identifies the organism
based on the unique proteomic pattern comparing
with an available, matching pattern in the
reference database. (8)
Disk diffusion
method was used for antifungal susceptibility
testing for azoles. Susceptibility test was done
using fluconazole(25µg) and voriconazole(1μg)
disks according to clinical laboratory standard
institute (CLSI)guidelines. (9,10) Antifungal
susceptibility for C. auris was
interpreted using C. albicans
breakpoints since reference zone diameters are not
available in CLSI or European Committee on
Antimicrobial Susceptibility Testing (EUCAST).
Data was entered in an Excel sheet and analyzed.
Categorical variables were expressed in terms of
frequencies and percentages and continuous
variables were expressed in terms of the median.
Results
During the study
period, a total number of 132 (0.74%) Candida
isolates from 17,721 blood cultures were isolated.
The patients were aged from 14 days to 90 (median
age, 52) with predominance of males (59%).
Majority were non- albicans Candida accounting
for 81.06 % of total candidemia isolates. Among
non-albicans Candida, C. tropicalis
(31.81%) was the most common candida
species isolated followed by C. parapsilosis
(20.45%). Isolation rate of C. auris was
13.63% in this study period and most of them were
from burns unit (58.3%) (Table 1)
|
Table 1: Species distribution of
Candida in candidemia patients
|
|
Candida spp
|
Number of isolates (Percentage)
|
|
C.tropicalis
|
42(31.81)
|
|
C.parapsilosis
|
27(20.45)
|
|
C.albicans
|
25(18.9)
|
|
C.auris
|
18(13.63)
|
|
C.orthopsilosis
|
11(8.33)
|
|
C.glabrata
|
5(3.78)
|
|
C.metapsilosis
|
1(0.75)
|
|
C.krusei
|
1(0.75)
|
|
C.nivariensis
|
1(0.75)
|
|
C.utilis
|
1(0.75)
|
|
Total
|
132(100)
|
Overall, fifty-nine
patients (44.69%) had diabetes mellitus as
associated morbidity. Two most common risk factors
identified in patients with candidemia were
concurrent antibiotic use (95.45%) and use of
intravenous(IV) device (51.51%). (Fig. 1)
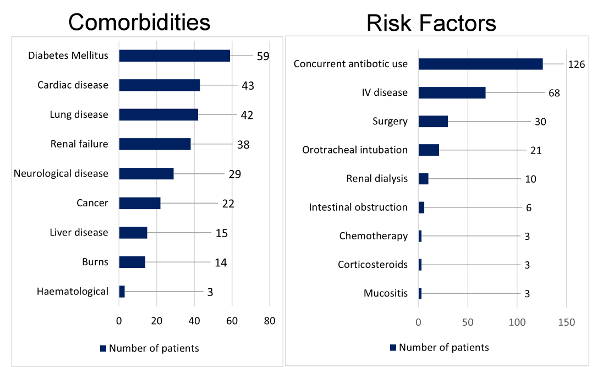
|
| Figure
1: Comorbidities and Risk factors for
candidemia |
Susceptibility to
both fluconazole and voriconazole was 100% for C.
albicans and C. tropicalis isolates.
Though the voriconazole susceptibility was
100% in C. parapsilosis, fluconazole
susceptibility was lower (88.23%). None of the C.
auris isolates were susceptible to
fluconazole though the voriconazole susceptibility
of 72% was observed.
Discussion
Candidemia is the
most common presentation of invasive infections by
candida species. Early detection and
targeted therapy are important in preventing fatal
outcome. Worldwide changing epidemiology of candida
species and their susceptibilities warrant the
clinicians and microbiologists for an accurate
identification of the candida species. (2,3,5)
Etiologies of
candidemia are shifting away from C. albicans
and heading towards Candida species
other than C. albicans which have a high
tendency for developing resistance, such as the
multidrug-resistant C. auris, which is
rapidly disseminating throughout the world.
Increased prevalence of non-albicans Candida
was observed in our study which is concordant with
few other studies from India (5, 6) Among non-
albicans Candida, C. tropicalis
was the most common isolate followed by C.
parapsilosis which is similar to the
findings from Singh et al and Abdel-Hamid et al
studies. (11,12)
There is a need to
assess patients who are at risk of acquiring candida
infection for the prevention of adverse outcome of
the patients and healthcare burden. Diabetes
mellitus and cardiac disease were the most common
comorbidities and concurrent antibiotic use was
the most common risk factor associated with
candidemia in our study which is concordant with a
study conducted Deepali et al (13).
We found a high rate
of isolation for C. auris (13.63%) in
our study which is very concerning. In a study
from western India showed C. auris as
the most frequent candida species
isolated from candidemia patients with the
isolation rate of 43%. (7) Burns patients with
impaired immune defense and large wounds are at
high risk of acquiring C. auris
infection from the hospital environment.(14) It is
important to identify C. auris
accurately so that infection control practices can
be followed strictly to control the spread of C.
auris strain in the hospital.
In our study, we
observed that the fluconazole was the most common
antifungal agent used in treatment with an average
duration of 2 weeks. Fluconazole with Caspofungin
was used for the treatment of candidemia patients
with C. auris. The recommended duration
of antifungal therapy for uncomplicated candidemia
cases should be extended to 2 weeks after the
documented clearance of candida from blood stream
and the resolution of symptoms. (15)
The increasing
isolation of non- albicans Candida,
especially high prevalence of C. auris
along with fluconazole resistance in non-albicans
Candida in this study is of concern.
Continuous evaluation is essential for those
patients with risk factors to predict the
development of candidemia and for the early
intervention. Our study findings highlight the
need of strengthening hospital infection control
practices and implementation of antimicrobial
stewardship program in order to prevent the rise
in antifungal resistance.
References
- Edwards JE. Candida species. In: Bennet JE,
Dolin R, Blaster MJ editors. Principles and
Practice of Infectious Diseases 9th ed.
Elsevier: Churchill Livingstone. 2020:
3087-3102.
- Mareković I, Pleško S, Rezo Vranješ V,
Herljević Z, Kuliš T, et al. Epidemiology of
Candidemia: Three-Year Results from a Croatian
Tertiary Care Hospital. Journal of Fungi.
2021; 7(4):267. https://doi.org/10.3390/jof7040267
- Meyahnwi D, Siraw BB, Reingold A.
Epidemiologic features, clinical
characteristics, and predictors of mortality in
patients with candidemia in Alameda County,
California; a 2017–2020 retrospective analysis.
BMC Infect Dis. 2022:843.
https://doi.org/10.1186/s12879-022-07848-8
- Ray A, Adarsh AK, Banerjee S, Chakrabarti A,
Denning DW, Burden of Serious Fungal Infections
in India. Open Forum Infect Dis.
2022;12(9):603.https://doi.org/10.1093/ofid/ofac603
- Kaur H, Singh S, Rudramurthy SM, Ghosh AK,
Jayashree M, Narayana Y, et al. Candidaemia in a
tertiary care center of a developing country:
Monitoring possible change in the spectrum of
agents and antifungal susceptibility. Indian
J Med Microbiol. 2020;38(1):110-116. doi:
10.4103/ijmm.IJMM_20_112.
- Gautam G, Rawat D, Kaur R, Nathani M.
Candidemia: Changing dynamics from a tertiary
care hospital in North India. Curr Med
Mycol. 2022;8(1):20-25. doi:
10.18502/cmm.8.1.9210.
- Prayag PS, Patwardhan S, Panchakshari S,
Prayag A. The Dominance of Candida auris:
A Single-center Experience of 79 Episodes of
Candidemia from Western India. Indian J Crit
Care Med. 2022; 26 (5):560-563.
- CLSI Methods for the identification of
cultured microorganisms using Matrix assisted
laser desorption ionization -time of flight mass
spectrometry. 1st ed. CLSI guidelines
M58. Wayne PA: Clinical and laboratory institute
2017.
- CLSI. Method for antifungal disk diffusion
susceptibility testing of yeasts. 3rd
ed. CLSI guideliLnes M44. Wayne PA: Clinical and
aboratory Institute 2018.
- CLSI. Performance standards for antifungal
susceptibility testing of yeasts.3rd
ed. CLSI supplement M27M44S. Clinical and
Laboratory Institute 2022
- Singh DP, Kumar Verma R, Sarswat S, Saraswat
S. Non-Candida albicans Candida
species: virulence factors and species
identification in India. Curr Med Mycol. 2021;7(2):8-13.
doi: 10.18502/cmm.7.2.7032.
- Abdel-Hamid RM, El-Mahallawy HA, Abdelfattah
NE, Wassef MA. The impact of increasing
non-albicans Candida trends on
diagnostics in immunocompromised patients. Braz
J Microbiol 2023;54: 2879–2892. https://doi.org/10.1007/s42770-023-01163-3
- Dixit D, Jen P, Maxwell TD et al. Risk Factors
and Clinical Outcomes of Candidemia Associated
with Severe COVID-19. Crit Care Explor. 2022
13;4(9): e0762. doi:
10.1097/CCE.0000000000000762.
- Moore R, Lie L, Pua H, Slade DH, Rangel S,
Davila A, et al. The Perfect Storm: A Hardy and
Lethal Pathogen and a Unit Filled with
Immunocompromised Patients with Large Open
Wounds. Troubles with Candida Auris in
a Burn Intensive Care Unit. Open Forum
Infect Dis. 2022; 9:492.1057. doi:
10.1093/ofid/ofac492.1057.
- Pappas PG, Kauffman CA, Andes DR et al.
Clinical Practice Guideline for the Management
of Candidiasis: Update by IDSA. Clin Infect
Dis. 2016;62(4): e1–e50.
|



















