|
Introduction
Breast
cancer (BC) is the most common cancer in women
worldwide and the leading cause of cancer related
mortality in women. (1) In India, it is second
most common malignancy after cancer cervix among
females. (2) BC is considered as a heterogeneous
disease sharing similar histopathologic features
but with distinctive clinical presentation,
biologic features, behaviour, outcome and response
to treatment, (3) which is attributed to molecular
diversity among histologically similar tumours.
(4) Hence, despite the progress in BC therapy,
there is no incisive therapy for BC treatment and
this highlights the importance to develop new
therapeutic strategies, alternative to the
currently used drugs, such as Tamoxifen or
Trastuzumab or Lapatinib, which are only useful
when the target proteins (Estrogen receptor or
Her2) are expressed. It is in this scenario, the
androgen receptor (AR) is emerging as a new marker
and a potential new therapeutic target in the
treatment of BC. (5,6)
Androgen receptor
belongs to the steroid hormone nuclear receptor
family similar to estrogen receptor (ER) and
progesterone receptor (PR). The role of AR in
development of prostate and progression of
prostate cancer is well documented. However, its
role in breast cancer is unclear (1,2)
and the precise understanding of its action
remains a challenging puzzle. (5) The role of AR
also depends on the tumor microenvironment as well
as the relative levels of circulating estrogens
and androgens. (5) It has been hypothesized that
androgen may influence breast cancer risk
indirecty through their conversion to estradiol or
by competing for steroid binding proteins, or
directly by binding to the AR. (1,2) Thus, AR is
thought to play a central role in its initiation,
progression of breast cancer, and its response to
therapy. (1)
Gene expression
profiling characterized four major groups of BC,
which classified patients into Luminal A, Luminal
B, HER-2/neu enriched, and Triple Negative BC
(TNBC); based on immunohistochemical staining for
Estrogen Receptor (ER), Progesterone Receptor
(PR), HER-2/neu and Ki-67 staining. (3,7) This
helped stratification of BC patients for
prognostic and therapeutic purposes. AR expression
in relation to different molecular sub-types of BC
is not clearly understood. (3) Currently,
investigators suggest that AR positive tumours
have favourable characteristics and that tumours
expressing both AR and ER are associated with
better outcome. (4) In studies by Hu et al.
and Agoff et al., AR expression and
patient's survival depend on the status of ER.
(8,9) Hence the coexpression of these receptors
may be needed to assess better prediction of
patient’s survival. (1)
Whatever be the
mechanism, AR stimulates or inhibits cellular
proliferation and promotes metastatization or
resistance to therapies in ER-positive BC cells.
In ER-negative BC cells, it clearly promotes cell
proliferation and spreading by acting at different
levels. This scenario makes possible the use of AR
modulators or blockers in BCs. (5) In ER-positive
BCs and in a subset of ER-negative BCs in which AR
activation inhibits tumor growth, natural and
synthetic steroidal androgens have been used for
therapeutic purpose. But these are known to induce
many side effects. Thus the use of selective AR
modulators (SARMs, i.e., enobosarm GTx-024) which
have less side effects with favorable results has
been considered for the therapy of ER-positive
advanced BCs patients. This is still investigated
in a phase II clinical trials in patients with ER
positive BC. (5)
The prognostic and
predictive value of AR in Triple negative breast
cancer (TNBC) remains a challenging topic of
research. There is a subtype of TNBC which
positively express AR termed as luminal androgen
receptor (LAR) subtype. The AR antagonist
(enzalutamide) implicated in prostate cancer
treatment, has shown promising results in some
patients with advanced TNBC whose tumours were
AR-positive. (4)
There is limited
literature from India, on role of AR in breast
cancer. (1) Hence this study was undertaken to
evaluate the AR expression in primary carcinoma
breast and to find its association with
clinic-pathological features, ER,PR, HER2 and Ki67
status and its molecular subtypes.
Materials and Methods
Patient variables
A retrospective
study was conducted in the department of
Pathology, during the year 2017-2022, after
approval from Institutional Ethics Committee. The
study included 64 cases of mastectomy specimens of
primary breast carcinoma in female patients with
known hormone receptor status (ER, PR, Her2) and
Ki 67 proliferation index. Patients with
inadequate clinical data or cases with unavailable
slides and blocks were excluded from the study.
Patient demographic details such as age,
laterality and histopathological parameters such
as histological type, tumor size, lymphovascular
emboli (LVI), grade of the tumor (Modified
Bloom‑Richardson grade), lymph node involvement,
metastasis, and hormone receptor status (ER, PR,
and Her‑2 receptor), Ki 67 index were retrieved
from the hospital laboratory information system
and medical records department. Molecular
classification of the tumor was done based on
hormone receptor status into Luminal A, Luminal B,
Her 2 enriched and basal type.
Immunohistochemistry
Immunohistochemistry
(IHC) staining for AR was performed on 4–5 μm
thickness tissue sections. The slides were
incubated overnight at 60°C. Antigen retrieval was
performed in citrate buffer using pressure cooker
method. The slides were incubated with primary
rabbit monoclonal antibody (clone EP120,
Pathnsitu) at room temperature for 30 min. The
slides were then incubated with secondary antibody
and using diaminobenzidine as chromogen,
immunoreactivity was detected. The slides were
counterstained with Harris’s hematoxylin. Prostate
tissue was used as AR‑positive controls. A
negative control with exclusion of primary
antibody was done done. Tumors with ≥10% nuclear
staining of neoplastic cells were considered as
positive. (1) Intensity was scored as 0 for no
staining, 1 for weak, 2 for moderate, and 3 for
strong intensity staining.
For ER and PR, tumor
cells with at least 1% stained cells were
considered as positive. Her‑2 status was
interpreted according to the American Society of
Clinical Oncology/College of American Pathologists
guideline recommendations. (9) Both the score of
1+ and 2+ were considered as negative.
Ki67 proliferative
index assessment was expressed as the percentage
of ki 67- positive cells within the total number
of malignant cells among five high-power fields
(×400). (10) A percentage of 14% or more was
considered to be a cutoff point. (3)
Molecular subtyping
of breast carcinoma was done using a combination
of four IHC markers (ER, PR, HER-2/neu, and Ki-67)
according to St. Gallen international expert
Consensus, 2013. (7)
Statistical
analysis
The collected data
were coded, tabulated, and statistically analyzed
using statistical Package for Social Sciences
version 26.0 software. Descriptive statistics were
done for quantitative data; The Chi-square test
was used to assess the association between
clinicopathological variables and AR positivity. A
value of P < 0.05 was considered as
statistically significant.
Results
AR expression was
noted in 67.2% (43/64) of tumors in this study
population. The relationship between various
clinicopathological parameters and biomarkers with
AR expression is depicted in Table 1.
|
Table 1: AR expression and its
association with various
clinicopathological parameters and
biomarkers.
|
|
Parameters
|
TOTAL (n=64), n(%)
|
AR + (n = 43) , n(%)
|
AR – (n=21), n(%)
|
P(AR+ vs AR-)
|
|
AGE
|
0.614
|
|
<50
|
21 (32.8)
|
15 (71.4)
|
6 (28.6)
|
|
>50
|
43 (67.2)
|
28 (65.1)
|
15 (34.9)
|
|
LATERALITY
|
0.934
|
|
LT
|
34 (53.1)
|
23 (67.6)
|
11 (32.4)
|
|
RT
|
30 (46.9)
|
20 (66.7)
|
10 (33.3)
|
|
HISTOLOGICAL TYPE
|
0.266
|
|
IDC
|
59 (92.2)
|
38 (64.4)
|
21 (35.6)
|
|
ILC
|
1 (1.6)
|
1 (100.0)
|
0 (00)
|
|
MUCINOUS CA
|
4 (6.2)
|
4 (100.0)
|
0 (00)
|
|
TUMOR SIZE
|
0.612
|
|
T1
|
7 (10.9)
|
5 (71.4)
|
2 (28.6)
|
|
T2
|
40 (62.5)
|
28 (70.0)
|
12 (30.0)
|
|
T3
|
14 (21.9)
|
9 (64.3)
|
5 (35.7)
|
|
T4
|
3 (4.7)
|
1 (33.3)
|
2 (66.7)
|
|
MBR GRADE
|
0.172
|
|
Grade I
|
14 (21.9)
|
12 (85.7)
|
2 (14.3)
|
|
Grade II
|
35 (54.7)
|
23 (65.7)
|
12 (34.3)
|
|
Grade III
|
15 (23.4)
|
8 (53.3)
|
7 (46.7)
|
|
LVI
|
0.799
|
|
Present
|
38 (59.4)
|
26 (68.4)
|
12 (31.6)
|
|
Absent
|
26 (40.6)
|
17 (65.4)
|
9 (34.6)
|
|
LYMPH NODE
|
0.673
|
|
N0
|
33 (51.6)
|
22 (66.7)
|
11 (33.3)
|
|
N1
|
15 (23.4)
|
9 (60.0)
|
6 (40.0)
|
|
N2
|
11 (17.2)
|
9 (81.8)
|
2 (18.2)
|
|
N3
|
5 (7.8)
|
3 (60.0)
|
2 (40.0)
|
|
ER
|
0.001
|
|
Positive
|
42 (65.6)
|
34 (81)
|
8 (19)
|
|
Negative
|
22 (34.4)
|
9 (40.9)
|
13 (59.1)
|
|
PR
|
0.010
|
|
Positive
|
33 (51.6)
|
27 (81.8)
|
6 (18.2)
|
|
Negative
|
31 (48.4)
|
16 (51.6)
|
15 (48.4)
|
|
HER2
|
0.485
|
|
Positive
|
21 (32.8)
|
16 (76.2)
|
5 (23.8)
|
|
Negative
|
39 (60.9)
|
24 (61.5)
|
15 (38.5)
|
|
Equivocal
|
4 (6.2)
|
3 (75)
|
1 (25)
|
|
KI 67
|
0.430
|
|
< 14 %
|
13 (20.3)
|
8 (61.5)
|
5 (38.5)
|
|
>14 %
|
51 (79.7)
|
35 (68.6)
|
16 (31.4)
|
|
LUMINAL A
|
0.649
|
|
Positive
|
6 (9.4)
|
4 (66.7)
|
2 (33.3)
|
|
Negative
|
58 (90.6)
|
39 (67.2)
|
19 (32.8)
|
|
LUMINAL B
|
0.002
|
|
Positive
|
36 (56.2)
|
30 (83.3)
|
6 (16.7)
|
|
Negative
|
28 (43.8)
|
13 (46.4 )
|
15 (53.6)
|
|
TRIPLE NEGATIVE
|
0.003
|
|
Yes
|
13 (20.3)
|
4 (30.8)
|
9 (69.2)
|
|
No
|
51 (79.7)
|
39 (76.5)
|
12 (23.5)
|
|
HER2 ENRICHED
|
0.329
|
|
POSITIVE
|
9 (14.1)
|
5 (55.6)
|
4 (44.4)
|
|
NEGATIVE
|
55 (85.9)
|
38 (69.1)
|
17 (30.9)
|
The patients ranged
in age from 31 to 88 years, (mean=56.4years) and
21 cases (32.8%) out of 64 patients were younger
than 50 years. 43 (67.2%) patients were above the
age of 50 years. AR expression (71.4%) was noted
in patients aged below 50 years and 65.1% in age
above 50 years. No statistically significant
association was noted between age and AR
expression.
Thirty four (53.1%)
cases has lump in the right breast and 30 (46.9%)
in left breast. Laterality did not have any
association with AR expression.
Majority 59/64
(92.2%) tumors were infiltrating duct carcinoma
(IDC), four cases (6.2%) being mucinous carcinoma
and one case (1.6%%) of invasive lobular carcinoma
(ILC). 64.4% of IDC showed AR expression. All the
mucinous carcinoma in this study showed AR
expression. But no significant statistical
association was noted with histological type of
tumor. AR expression of various intensities in
carcinoma breast is depicted in (Fig 1).
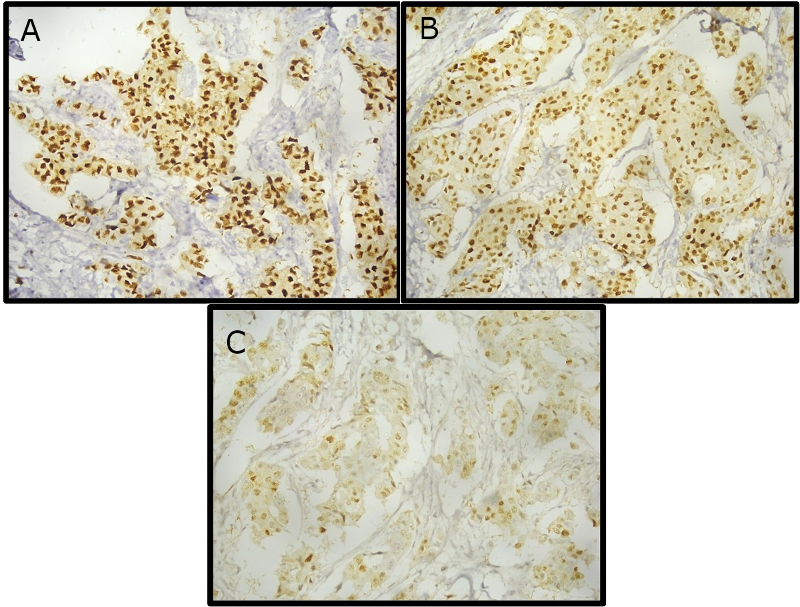
|
| Fig
1: AR expression of various staining
intensities in carcinoma breast. A-Strong
intensity (Score 3), B- Moderate intensity
(Score 2), C- Weak intensity (Score 1).
|
Out of 64 studied
cases, 14 (21.9%) were grade I, 35 (54.7%) grade
II, and 15 (23.4%) cases were grade III tumors. AR
was positive in 12 (85.7%) of grade I, 23 (65.7%)
of grade II, and 8 (53.3%) of grade III tumors. A
higher AR positivity was noted in grade 1 and 2
tumors but was not statistically significant. No
association between tumor grade and AR expression
was identified
Among the
histopathologic parameters evaluated, tumor size,
LVI, lymph nodal status and metastasis did not
show any association with AR expression.
Expression of ER, PR
and Her‑2 receptors was noted in 65.6% (n = 42),
51.6% (n = 33), and 32.8% (n = 21), respectively.
Ki 67 expression was <14% in 20.3% (n=13) and
>14% in 79.7% (n= 51) cases. AR was expressed
in 81% of ER‑positive tumors and 40.9% in ER
negative tumor. AR expression was noted in 81.8%
of PR positive tumors 51.6% of PR negative tumor.
AR expression showed a significant association
with ER (P = 0.001) and PR status of the tumors (P
= 0.010). In Her‑2 positive tumors, AR expression
was seen in 76.2% of the tumors. In spite of
higher percentage of AR positivity in Her‑2
positive tumors, the P value was not statistically
significant (p=0.329).
Among luminal A,
luminal B, Her‑2 overexpression, and
triple‑negative cancers, the rates of AR
expression were as follows: 66.7%, 83.3%, 30.8%,
and 55.6%, respectively. There was significant
association between AR positive expression with
Luminal B tumors (P = 0.002). AR expression was
noted in 30.8% of triple negative tumors and was
considered statistically significant (P = 0.003).
Discussion
Over the past two
decades, hormone receptors (ER/PR) and Her‑2
growth factor receptors biomarkers have gained
importance due to implications in prognosis and
clinical management. (1) Several studies have
examined the correlation of ER and PR with other
prognostic indicators, but little is known about
the role of AR and its prognostic value in breast
carcinoma. (2)
Current concept is
that different types of luminal cells in the
normal breast epithelium might serve as a
precursor for different subtypes of breast cancer.
AR expression is found in luminal and metaplastic
apocrine cells. Even through AR is expressed in
the luminal cells, they are not biologically
active. (1) Wang reported that there is an absence
of downstream regulatory proteins
(prostate‑specific antigen, gross cystic disease
fluid protein) in the normal breast epithelium by
IHC. Thus, further oncogenic events are required
for the initiation and progression of breast
cancer in these AR‑positive luminal cells. (11)
AR is highly
expressed in breast cancer. The positive rates of
expression of AR vary mostly from 60% to 80% in
the literature. (1) Our study showed AR expression
rate of 67.2% which was almost similar as compared
to the Western literature and also study done by
Ismael et al. (4) This varied AR expression rates
may be due to the different methodology used and
the geographical distribution of the population
studied. (1)
The relationship
between the AR expression status and the various
clinicopathological parameters showed inconsistent
results in literature published. The grade of the
tumor is the most consistent parameter which
correlated with AR status. Park et al. reported
that, patients with smaller tumor size and lower
histological grade had a higher AR expression, but
not with age, menopausal status, body mass index,
nodal involvement, preoperative CEA levels, and
stage. (12) Agrawal et al. found a relation with
only tumor grade. (13) On the other hand, Gonzalez
et al. (14) and Samaka et al., (3) found no
significant correlation between AR expression and
the size of the tumour. Similar to study done by
Ismael et al,(4) this study also found no between
AR status and patients age, laterality, tumour
size, histologic type, grade, lymphovascular
invasion, T stage, N stage, Her-2/neu status and
ki 67 status.
The frequency of AR
expression is generally comparable with or higher
than that of ER/PR expression. (1) In this study,
the expression rates AR showed a significant
association with ER (P = 0.001) and PR status of
the tumors (P = 0.010). A significant correlation
between AR expression and hormonal status was also
found by Park et al.,(12) Qi et al., (15)
Safarpour et al., (16) Vera-Badillo et al., (17)
and Chottanapund et al.. (18) AR was expressed in
81% of ER positive tumors and 81.8% of PR positive
tumors. In ER negative patients, AR is present in
40.9% of cases. Hence ER negative cases not
responding to treatment are treated with
medroxyprogesterone acetate which mediates its
action by binding to AR. (2)
In our study, in
spite of higher percentage of AR positivity in
Her‑2 positive tumors, the P value was not
statistically significant (p=0.329). Park et al.,
(12) and Qi et al., (15) found no significant
relation between AR expression and Her-2/neu
expression status, although Agrawal et al., (13),
Chottanapund et al., (18) and Samaka et al., (3)
found that AR expression is more in tumours
expressing Her-2/neu. This could be due to
different primary antibodies used.
Our study showed no
significant relation between AR expression and Ki
67 expression similar to study done by Vera-
Badillo et al., (17) in contrast to Qi et al.,
(15) and Samaka et al. (3) this might be due
different cut-offs used.
ER, PR, and Her‑2
have been considered as immunohistochemical
surrogate markers for molecular subtypes of
cancer.(1) Regarding the correlation between AR
expression and the molecular subtypes, AR
expression was seen significantly higher in
luminal breast carcinoma cases. This is by Collins
et al., (19) Qi et al., (15) and Samaka et al. (3)
In triple‑negative
tumors, the rates of AR expression varied from
6.6% to 75%. (20) In this study, AR expression in
triple negative patients was 30.8% and it showed
significant association with AR expression (p<
.003) which was similar to study done by Ismael et
al. (4) These subsets of patients are possible
candidates for the promising anti-androgen target
therapy. (4) Studies state that androgen can
inhibit the growth of hormone negative breast
cancer if there is strong expression of AR,
because of conversion of androgen to estrogen by
the aromatase enzyme. These androgens can also
induce apoptosis regardless of ER and PR status.
Thus, AR antagonist may be used in AR positive
tumours regardless of their ER status. (2)
The strength of this
study is that it has been done in a population of
patients in whom the clinicopathological data
regarding the role of AR expression is sparse. The
limitations of this study are small sample size
and that AR expression could not be correlated
with overall survival and disease‑free survival as
patients could not be followed up.
Conclusion
We conclude that
breast cancer has significant AR expression which
is significantly associated with ER and PR but not
with HER2/neu status. AR is also significantly
expressed in Luminal B subtype and triple negative
cases. Also, a subset of TNBC cases showed
significant positive AR expression. Further
researche on AR expression in breast cancer are
recommended on a larger scale with follow up and
survival to validate the current results and to
determine whether AR could be a therapeutic target
in triple negative and other molecular subtype of
breast cancer patients.
References
- Vellaisamy G, Tirumalae R, Inchara Y.
Expression of androgen receptor in primary
breast carcinoma and its relation with
clinicopathologic features, estrogen,
progesterone, and her-2 receptor status. J
Cancer Res Ther. 2019;15(5):989.
- Saxena AK, Agarwal M, Singhal J, Gupta A.
Evaluation of Androgen Receptor in Breast
Carcinoma and its Correlations with Er, Pr,
Her2/Neu, Triple Negative Receptor Status and
Clinical Parameters. Int Arch Biomed Clin
Res. 2017;3(3).
- Samaka RM. Androgen Receptor Expression in
Breast Carcinoma of Egyptian Patients. J
Clin Diagn Res. 2016; 10(11).
- Ismael NEHS, Khairy RA, Talaat SM, Abd
El-Fattah FA. Immunohistochemical Expression of
Androgen Receptors (AR) in Various Breast Cancer
Subtypes. Open Access Maced J Med Sci. 2019
Apr 29;7(8):1259–65.
- Giovannelli P, Di Donato M, Galasso G, Di
Zazzo E, Bilancio A, Migliaccio A. The Androgen
Receptor in Breast Cancer. Front Endocrinol.
2018 Aug 28;9:492.
- Bianchini G, Balko JM, Mayer IA, Sanders ME,
Gianni L. Triple-negative breast cancer:
challenges and opportunities of a heterogeneous
disease. Nat Rev Clin Oncol. 2016
Nov;13(11):674–90.
- Goldhirsch A, Wood WC, Coates AS, Gelber RD,
Thürlimann B, Senn HJ. Strategies for
subtypes—dealing with the diversity of breast
cancer: highlights of the St Gallen
International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann
Oncol. 2011 Aug;22(8):1736–47.
- Hu R, Dawood S, Holmes MD, Collins LC, Schnitt
SJ, Cole K, et al. Androgen Receptor Expression
and Breast Cancer Survival in Postmenopausal
Women. Clin Cancer Res. 2011 Apr
1;17(7):1867–74.
- Wolff AC, Hammond MEH, Allison KH, Harvey BE,
Mangu PB, Bartlett JMS, et al. Human Epidermal
Growth Factor Receptor 2 Testing in Breast
Cancer: American Society of Clinical
Oncology/College of American Pathologists
Clinical Practice Guideline Focused Update. Archives
of pathology & Laboratory Medicine.
2018 Nov 1;142(11):1364-82.
- Abdelaal SE, Gabal SM, Gamal El Din AA, Hosni
HN, Sharaf HA. Immunohistochemical Study of
Androgen Receptor Expression in Estrogen
Receptor-Negative Invasive Breast Carcinoma and
its Relation with Clinicopathologic Factors. Open
Access Maced J Med Sci. 2020 Sep
10;8(A):615–22.
- Wang xi. Androgen Receptor (AR) and Breast
Cancer: Reference to the AR Status in
Normal/Benign Breast Luminal Cells. Recept
Clin Investig. 2015;2:e533
- Park S, Koo J, Park HS, Kim JH, Choi SY, Lee
JH, et al. Expression of androgen receptors in
primary breast cancer. Ann Oncol. 2010
Mar;21(3):488–92.
- Agrawal A, Ziolkowski P, Grzebieniak Z, Jelen
M, Bobinski P, Agrawal S. Expression of Androgen
Receptor in Estrogen Receptor–positive Breast
Cancer. Appl Immunohistochem Mol Morphol. 2016
Sep;24(8):550–5.
- Gonzalez LO, Corte MD, Vazquez J, Junquera S,
Sanchez R, Alvarez AC, et al. Androgen receptor
expresion in breast cancer: Relationship with
clinicopathological characteristics of the
tumors, prognosis, and expression of
metalloproteases and their inhibitors. BMC
Cancer. 2008 Dec;8(1):149.
- Qi J ping, Yang Y lin, Zhu H, Wang J, Jia Y,
Liu N, et al. Expression of the Androgen
Receptor and its Correlation with Molecular
Subtypes in 980 Chinese Breast Cancer Patients.
Breast Cancer Basic Clin Res. 2012
Jan;6:BCBCR.S8323.
- Safarpour D, Pakneshan S, Tavassoli FA.
Androgen receptor (AR) expression in 400 breast
carcinomas: is routine AR assessment justified?
American Journal of Cancer Research. 2014;4(4):353.
- Vera-Badillo FE, Templeton AJ, De Gouveia P,
Diaz-Padilla I, Bedard PL, Al-Mubarak M, et al.
Androgen Receptor Expression and Outcomes in
Early Breast Cancer: A Systematic Review and
Meta-Analysis. JNCI J Natl Cancer Inst.
2014 Jan 1;106(1):djt319–djt319.
- Chottanapund S, Van Duursen M, Ratchaworapong
K, Navasumrit P, Ruchirawat M, Van Den Berg M.
Androgen Receptor Expression in Thai Breast
Cancer Patients. Med Sci. 2016 Sep
14;4(3):15.
- Collins LC, Cole KS, Marotti JD, Hu R, Schnitt
SJ, Tamimi RM. Androgen receptor expression in
breast cancer in relation to molecular
phenotype: results from the Nurses’ Health
Study. Mod Pathol. 2011
Jul;24(7):924–31.
- Rampurwala M, Wisinski KB, O’Regan R. Role of
the Androgen Receptor in Triple-Negative Breast
Cancer. 2017. Clinical Advances in
Hematology & Oncology: H&O. 2016
Mar;14(3):186.
|



















