|
Introduction
As
males age, the prostate becomes one of the most
common organs to be affected, accounting for
significant mortality and morbidity. It can
present with a wide spectrum of lesions, such as
hyperplasia, atrophy, inflammation, metaplasia,
premalignant lesions, and malignancy. While a
pathologist must be well versed in the features of
malignancy, it is also essential to identify and
get familiarised with the various benign lesions
of the prostate, which are a handful. Most of them
coexist with each other.
The numerous benign
alterations can mimic carcinomas and premalignant
lesions. Thus, making the histopathological
diagnosis of prostate adenocarcinoma challenging.
Furthermore, both benign mimickers and
adenocarcinoma can occur in the same age group.
Therefore, it is important to prevent incorrect
interpretation since it could lead to serious
implications.
The lesions that are
most often misdiagnosed as cancer are atrophy and
its variants, including simple atrophy, partial
atrophy, and postatrophic hyperplasia due to their
high nuclear to cytoplasmic ratio, infiltrative
nature, and inconspicuous basal cell layer.[1]
Benign hyperplastic lesions are a group with
proliferating glands and no atypia.[1] Among
these, the architecture of adenosis is most
alarming since it is composed of small, crowded
glands with a fragmented basal cell layer.[1] A
variant of basal cell hyperplasia with prominent
nucleoli can also create confusion since it
presents small dark glands, variable nuclear
atypia, and prominent nucleoli.[1] Benign clear
cell cribriform hyperplasia can mimic Gleason
grade 4 prostatic adenocarcinoma, which also forms
glands in the cribriform arrangement.
Among the
metaplasia, florid mucinous metaplasia can mimic
the rare foamy variant prostatic adenocarcinoma,
where both show the presence of characteristic
abundant foamy cytoplasm and minimal cytologic
atypia. Nephrogenic metaplasia can mimic signet
ring cell carcinoma of Gleason grade 5 when the
tubules of metaplastic glands are lined by hobnail
cells with prominent nucleoli.[2] Sometimes, the
small lumens may also contain blue mucin,
mimicking Gleason grade 3 prostatic
adenocarcinomas.[2]
Clinically, in the
aging male population, benign enlargement of the
prostate gland compresses the urethra and causes
urinary obstruction with symptoms such as
increased urinary frequency, hesitation,
dribbling, and incomplete emptying of the
bladder[3], while carcinoma comes with its
challenges! The development of histologic features
of BPH is dependent on the bioavailability of
testosterone and its metabolite,
dihydrotestosterone.[4]
Globally, prostate
cancer is the second most frequently diagnosed
cancer and the fifth leading cause of cancer death
in men.[5] In a populated country like India, it
constitutes about 5% of all malignancies in
males.[6] The prevalence of BPH increases from 20%
at 40 years of age to about 90% by the eighth
decade of life![7] Prostate-specific antigen
(PSA), digital rectal examination, and transrectal
ultrasound are important tools for evaluation.[8]
However, biopsy remains the gold standard for
final diagnosis.[8]
Although we have a
limitation due to the lack of
immunohistochemistry, we took a keen interest in
conducting a study on the benign mimickers of
prostate adenocarcinoma relying solely on
morphology, the fundamental skill of a
pathologist. It has been challenging but fruitful
in improving our knowledge and observation skills.
We also did not come across many studies on the
web, that are focused entirely on the numerous
benign histopathological lesions. Hence, this
study is conducted considering the features of
various benign lesions, highlighting the mimickers
of carcinoma, and comparing their prevalences.
Materials and Methods
This retrospective
study was done in the Department of Pathology of
Veer Chandra Singh Garhwali Government Institute
of Medical Sciences for a period of two years,
i.e., from January 2022 to December 2023, after
approval from the Institutional Ethical Committee.
A total of 173 radical prostatectomy specimens
were received during this period.
The
histopathological data maintained in the
department of pathology were reviewed.
Haematoxylin and eosin (H&E) stained sections
were re-examined. New H&E-stained paraffin
sections were made whenever required, such as in
the case of faded slides, and re-staining and
re-mounting were done whenever required. All
relevant clinical details were reviewed from the
respective requisition forms submitted to the
Department of Pathology. The various prostate
lesions were listed, and the data was expressed
numerically and in percentages. Photomicrographs
of the benign lesions were taken.
Results
During the two-year
study period from January 2022 to December 2023,
the Department of Pathology received 173 radical
prostatectomy specimens. Of these, 156 were
diagnosed as benign lesions and 17 as malignant. A
variety of benign histopathological features were
observed and identified. They are listed in Table
1.
The most common
alteration found was Basal cell hyperplasia, which
was present in 66% of the cases, and the least
common was Nephrogenic metaplasia, which was found
in 3% of the cases.
|
Table 1: Histopathological features of
benign prostate lesions
|
|
Histopathological feature
|
No. of Cases
|
Percentage
|
|
1.
|
Acute inflammation
|
65
|
41.66
|
|
2.
|
Chronic inflammation
|
87
|
55.76
|
|
3.
|
Granulomatous inflammation
|
9
|
5.76
|
|
4.
|
Basal cell hyperplasia
|
104
|
66.66
|
|
5.
|
Clear cell cribriform hyperplasia
|
70
|
44.87
|
|
6.
|
Leiomyomatous hyperplasia
|
48
|
30.76
|
|
7.
|
Adenosis (atypical adenomatous
hyperplasia)
|
60
|
38.46
|
|
8.
|
Partial atrophy
|
52
|
33.33
|
|
9.
|
Simple atrophy
|
69
|
44.23
|
|
10.
|
Sclerotic atrophy
|
34
|
21.79
|
|
11.
|
Postatrophic hyperplasia
|
60
|
38.46
|
|
12.
|
Squamous metaplasia
|
51
|
32.69
|
|
13.
|
Mucinous metaplasia
|
34
|
21.79
|
|
14.
|
Transitional cell metaplasia
|
62
|
39.74
|
|
15.
|
Paneth cell-like change
|
25
|
16.02
|
|
16.
|
Nephrogenic metaplasia
|
5
|
3.20
|
|
17.
|
HGPIN
|
19
|
12.17
|
These histopathological features have been
broadly classified into five categories for
comparison. The categories are hyperplasia,
metaplasia, atrophy, inflammation, and
premalignant lesion, high grade prostatic
intraepithelial neoplasia, referred to as HGPIN.
Among the hyperplastic lesions, the most common
was Basal cell hyperplasia constituting 66% of the
cases.
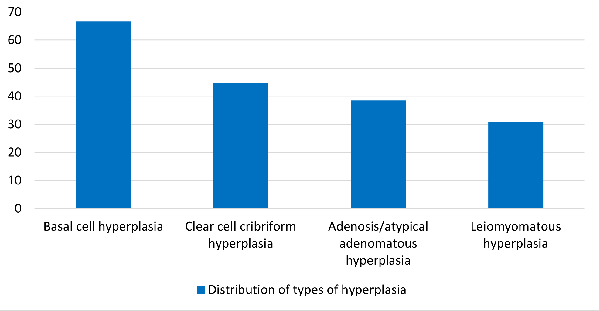
|
Graph
1: Hyperplastic lesions
|
The hyperplastic lesions are plotted in Graph 1.
We have found the three types of inflammation
mentioned in various literatures. They are acute,
chronic, and granulomatous. Among these, chronic
inflammation was the most common with a percentage
of 55%.
A variety of metaplastic lesions were observed in
our study. Out of these, transitional metaplasia
was the most common with a percentage of 39%.
These are shown in Graph 2.
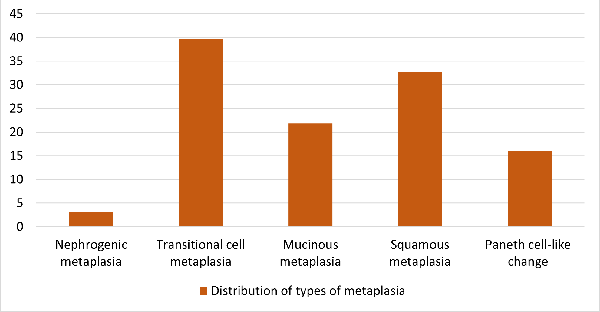
|
| Graph
2: Metaplastic lesions |
The various types of atrophy found are plotted in
Graph 3. Simple atrophy was the most common which
comprised 44% of cases.
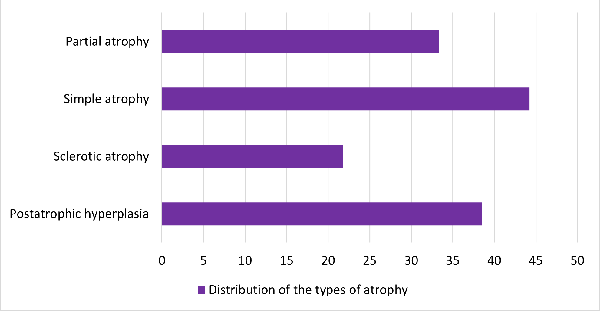
|
| Graph
3: Atrophic lesions |
The only premalignant lesion found was High-Grade
Prostatic Intraepithelial Neoplasia (HGPIN), which
accounted for 12% of all benign cases. Although
some of the slides did show features favouring
Low-grade Prostatic Intraepithelial Neoplasia
(LGPIN), it was not reported since LGPIN is highly
subjective, does not hold any pathologic
significance, and lacks clinical relevance.[9]
The age range in our study was 49 – 85, with the
maximum number of cases in the 7th decade of life.
The age-wise distribution of cases is shown in
Graph 4.
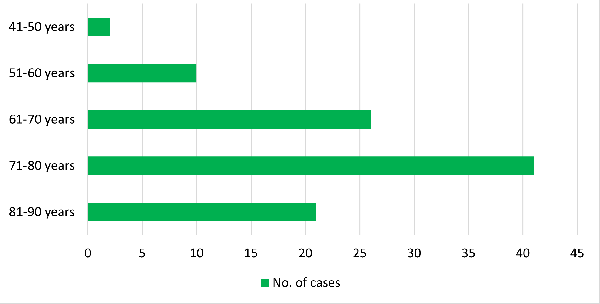
|
| Graph
4: Age-wise Distribution of Cases |
Discussion
Diseases of the
prostate are one of the most common causes of
decreased quality of life in the elderly male
population. They can cause urinary symptoms and,
in turn, discomfort to aging males. Being able to
distinguish benign mimics from prostatic
adenocarcinoma is of prime importance since an
incorrect diagnosis will cause trouble for the
patient and the doctor.
In our study, 156
prostate specimens were analysed that were
diagnosed as benign lesions. The age of the
patients ranged from 49 – 85 years, with the
maximum number of cases in the 7th
decade of life. Albasri et al. also found
a peak incidence of benign lesions in the age
range of 70-79.[10]
This supports the
fact that the frequency of prostatic lesions
increases as males grow older, with a high risk
for someone who has a family history of prostate
disease thereby revealing an underlying genetic
cause. In older males, testosterone starts to
decline, dihydrosterone increases, and oestrogen
remains the same. These hormonal changes bring
about an increase in growth factors, leading to
epithelial and stromal proliferation. Prostate
lesions are also associated with a lack of
physical activity, obesity, type 2 diabetes,
erectile dysfunction, and cardiovascular disease.
Most of these factors are common among the older
males.
Among
inflammation,chronic inflammation was found to be
the most common in our study. This concurs with
the study done by Pushpa et al., where
it comprised 40% of the cases.[11]
On microscopy,
lymphoplasmacytic infiltrates are present around
the glands and in the stroma. Lymphocytes may show
perinuclear clearing, appearing as signet ring
cells. This can mimic Gleason pattern 5 prostatic
adenocarcinoma which can exhibit signet ring cell
change.[12] Careful observation of the glandular
nuclear features is key. Chronic inflammation is
believed to be multifactorial. These include
previous infection of the genitourinary system,
instrumentation, immune system dysfunction,
psychological stress, and irregular hormone
activity, which are common in the aging male
population.
Acute inflammation
is associated with various nonneoplastic
epithelial alterations, such as atrophy,
hyperplasia, squamous metaplasia, and transitional
metaplasia.[1,3] It is almost always associated
with benign lesions and rarely seen with
malignancy.[13]
Histologically, we
see sheets of neutrophils in and around the
glands, and in the stroma. Most cases of acute
inflammation are caused by bacteria responsible
for other urinary tract infections, including Escherichia
coli (80% of infections), other Enterobacteriaceae,
Pseudomonas, Serratia, Klebsiella,
and Enterococci.[14]
Granulomatous
inflammation occurs due to several reasons. They
can occur following procedures, due to infectious
agents, and systemic disease but mostly it is
nonspecific.[15] The nonspecific type is a
reaction to destroyed prostatic ducts/acini from
which bacterial toxins, cellular debris, and
glandular secretions spill into the stroma.[16]
Post-procedural granulomatous reaction is the
second most common cause of granulomatous
inflammation and may be seen following
transurethral resections or needle core
biopsies.[15] Infectious agents involved are
Bacillus Calmette-Guerin (BCG)-related therapy,
bacterial, fungal, parasitic, and viral
pathogens.[15] Systemic causes include
Sarcoidosis, Wegener granulomatosis, Churg-Strauss
syndrome, and allergic prostatitis.[17]
In the nonspecific
variant, there are dilated ducts and acini filled
with histiocytes, foamy macrophages, and rarely
multinucleated giant cells.[18] Stroma comprises
epithelioid histiocytes and occasional
multinucleated giant cells.[18] In the infectious,
postsurgical, and systemic variants, multiple
granulomas with or without necrosis are seen,
along with histiocytes and giant cells.[18] It is
quite common to find a mixed inflammatory
infiltrate comprising neutrophils and lymphocytes
in all the variants. Eosinophils are found in
allergic prostatitis.[18]
In prostatic
adenocarcinoma post-therapy, tumor cells are
single with foamy cytoplasm and less nucleolar
prominence.[19] This mimics lipid-laden
epithelioid macrophages. Identifying the residual
glands in such cases is important, too.[19]
Diffuse granulomatous inflammation may mimic
high-grade prostatic adenocarcinoma.[19] Taking
note of nucleolar prominence in carcinoma is
important.
Basal cell
hyperplasia (BCH)was the most common hyperplastic
lesion found in our study, which is similar to the
study done by Mahapatra et al.[20] The
presence of nodular proliferation of glands with
multiple layers of basal cells having scant
cytoplasm gives the glands a basophilic
appearance. Intraluminal eosinophilic amorphous
secretions and cribriform architecture may be
seen. Nuclei are crowded with pinpoint nucleoli.
At higher magnification, basal cells are seen
oriented both parallel and perpendicular to the
basement membrane.[21]
Florid BCH, BCH with
prominent nucleoli, and BCH with cribriform
pattern must be distinguished from adenocarcinoma.
BCH is basophilic due to the multilayering of
basal cells and scant cytoplasm. It can present as
solid nests and lack nuclear pleomorphism.
Adenocarcinoma has an irregular arrangement of
cells, with relatively more abundant cytoplasm,
giving an eosinophilic or amphophilic appearance.
It exhibits pleomorphism and prominent nucleoli.
BCH, with prominent nucleoli, mimics HGPIN as
well. Its cells are perpendicular and parallel to
the basement membrane, with frequent solid nests
and uniform nuclei. On the contrary, HGPIN shows
crowding of large pleomorphic nuclei. BCH occurs
either due to a primary response to the luminal
epithelial apoptosis or a secondary response to
inflammation.[22] It also occurs following
androgen deprivation therapy and radiation
therapy.[23]
Adenosis, formerly
called atypical adenomatous hyperplasia, comprises
a nodular, well-circumscribed proliferation of
small, crowded glands with pale, abundant
cytoplasm without prominent nucleoli. The
flattened basal cell layer is partially intact and
fragmented, which may be difficult to visualize.
Adenosis mimics
low-grade prostatic adenocarcinoma, which also
comprises small, crowded glands. Carcinoma has
enlarged pleomorphic nuclei with prominent
nucleoli. It also has straight luminal borders and
a more infiltrative growth pattern. It lacks
larger, classic-appearing nonneoplastic glands
that are often intermixed with benign
adenosis.[24]
In clear cell
cribriform hyperplasia, hyperplastic prostatic
glands show cribriform architecture and contain
pale cytoplasm. Lumens are of varying sizes. The
basal cell layer is intact and prominent. Nuclei
are small and uniform with pinpoint nucleoli.
Prostatic adenocarcinoma Gleason pattern 4 variant
also appears to have a cribriform pattern but with
enlarged pleomorphic nuclei, prominent nucleoli,
and absence of basal cells.[25 ]
Leiomyomatous
hyperplasia is when there is increased
proliferation of stromal cells with minimal
glandular proliferation. The glands are compressed
by the increasing stroma. Bland fibromuscular
spindle cells, small blood vessels, and a myxoid
or hyalinised stroma form stromal nodules.
The morphological
differences between clear cell cribriform
hyperplasia, adenosis, and prostatic
adenocarcinoma have been enumerated in Table 2.
|
Table 2: Morphological differences
between clear cell cribriform hyperplasia,
adenosis, and carcinoma.
|
|
Characteristics
|
Clear Cell Cribriform Hyperplasia
|
Adenosis
|
Carcinoma
|
|
Architecture
|
Large normal-sized glands with cribriform
pattern.
|
Well-circumscribed, lobular, small
crowded glands.
|
Diffused, disordered, and infiltrative
glands. (Gleason grade 4 form cribriform
pattern; Gleason grade 3 form well-formed
glands with lumen)
|
|
Cytoplasm
|
Clear cytoplasm.
|
Abundant, pale eosinophilic to clear.
|
Mostly amphophilic, can be eosinophilic.
|
|
Nucleus
|
Small, uniform, round.
|
Small, uniform, round.
|
Large, pleomorphic crowding.
|
|
Nucleoli
|
Inconspicuous to Pinpoint.
|
Inconspicuous to Pinpoint.
|
Enlarged, prominent.
|
|
Basal cell layer
|
Prominent, continuous.
|
Fragmented, patchy.
|
Absent.
|
|
Inflammation
|
Common.
|
Common.
|
Rare.
|
|
Corpora Amylacea
|
Common.
|
Common.
|
Rare.
|
|
Blue tinged mucin
|
Rare.
|
Rare.
|
Common.
|
According to an
article by Bostwick et al., the
incidence of isolated HGPIN averages 9% (range
4%-16%) of prostate biopsies in the United States
every year.[26] In our study, HGPIN comprised 12%
of the cases during the 2-year time frame.
High-grade prostatic
intraepithelial neoplasia,orHGPIN, is a
premalignant lesion with cytological and
architectural atypia. Epithelial cells
proliferate, comprising nuclear and nucleolar
abnormalities.
On microscopy, four
architectural patterns can be seen. They are
tufting, micropapillary, cribriform, and flat.
Under low power, the glands appear darker and more
basophilic than normal. Layers of crowded,
pseudostratified secretory cells show enlarged and
irregular nuclei, hyperchromasia, and prominent
nucleoli at 20× magnification.[27] Chromatin is
coarse and clumpy.[27]
HGPIN is more common
in the peripheral zone of the prostate and is
often located adjacent to the foci of cancer.[27]
The presence of a basal cell layer differentiates
it from adenocarcinoma. Transitional cell
metaplasia has nuclear grooves which helps to
distinguish it from HGPIN.
HGPIN is more common
in men with prostate cancer.[27] Men with HGPIN on
initial core biopsy have a higher risk of prostate
carcinoma in the subsequent biopsy as compared to
those without HGPIN.[27] Carcinoma is most likely
to develop within 10 years of HGPIN.[26] HGPIN and
adenocarcinoma share the same causative factors,
including excess dietary fat, androgens, chronic
inflammation, and genetic mutations.[28] Loss of
p27 in HGPIN ultimately causes localized prostatic
adenocarcinoma.[28] Figure 1 shows the
morphological features of basal cell hyperplasia,
clear cell cribriform hyperplasia, adenosis, and
HGPIN.

|
| Figure
1: (a) Photomicrograph showing
basal cell hyperplasia of prostate glands
(40x, H&E). Inset shows a prostate
gland with multilayered basal cells (400x,
H&E). (b) Photomicrograph of prostate
glands showing cribriform architecture
(100x, H&E). Inset shows a gland with
clear cells arranged in cribriform pattern
(400x, H&E). (c) Photomicrograph of
adenosis showing small crowded glands
(100x, H&E). (d) Photomicrograph of
HGPIN with flat architecture (100x,
H&E). Inset shows tufted HGPIN with
pleomorphic nuclei and prominent nucleoli
(400x, H&E). |
Although not many
studies have encountered a wide range of
metaplasia, Abdollahi et al. conducted a
study where transitional cell metaplasia showed
the highest incidence, similar to ours.[29]
In transitional cell
metaplasia, transitional (urothelial) cells line
prostatic ducts or glands.[30] Glands may show a
spectrum of changes like that in the bladder,
including von Brunn’s nests and cystitis cystica
with punched-out lumens.[30] Lumens often contain
corpora amylacea. The glands have a multilayered
epithelial lining that imparts a basophilic
appearance. The cytoplasm is minimal and pale.[30]
Perinuclear clearing and longitudinal nuclear
grooves are present.[30]
Transitional cell
metaplasia can mimic high-grade prostatic
intraepithelial neoplasia. In contrast to
transitional cell metaplasia, HGPIN comprises
cytological atypia with prominent nucleoli and
lacks nuclear grooves. HGPIN also shows an array
of architectural patterns that are not present in
metaplasia.
It is induced by
tissue damage and associated with inflammation,
infarction, and post-therapy.[30] It is often
associated with a previous local irritation or
trauma due to surgical resections,
instrumentation, or stones.[2] In some cases, it
may occur after renal transplantation.[2] Such
situations are most prevalent in aging males.
Squamous metaplasia
is when squamous cells line prostate glands or
ducts.[30] It is also induced by tissue damage and
related to inflammation, infarction, radiation
therapy, and androgen deprivation therapy.[30 ]It
shows the presence of intercellular bridges, dense
eosinophilic cytoplasm, and inflammatory cell
infiltrate.
It should be kept in
mind that squamous cell carcinoma of the prostate
is a rare entity.[31] In carcinoma, the nucleus
shows marked atypia, hyperchromasia, and abnormal
mitotic figures. These nuclear features are not
found in metaplasia. Carcinoma is also not
associated with areas of infarction or
inflammation.[31]
In Mucinous
metaplasia, the prostate glands have mucin-filled
apical cells. The acinar cells are columnar,
containing abundant clear to blue cytoplasm.
Nuclei are pyknotic, small, and round, but mostly
it is flattened. As the acinar cell nuclei are
pushed by mucin towards the basal surface, basal
cells may be difficult to visualize without
immunohistochemistry.[32]
The extravasation of
mucin into the lumens may raise concern, as this
is a feature of adenocarcinoma. One must look for
the presence of basal cells, whether or not there
is an infiltrative growth pattern, and the
presence of more classic benign appearing prostate
glands to differentiate it from foamy-variant
prostatic adenocarcinoma. Although this variant of
prostatic adenocarcinoma is rare, it shows minimal
to no cytological atypia and pink luminal
secretions. It also comprises small hyperchromatic
nuclei which may make it difficult to visualise
the nucleoli. In such cases, special stains may be
required. The foamy variant of adenocarcinoma is
positive for colloidal iron, alcian blue, and
P504S[33] whereas mucinous metaplasia is positive
for mucicarmine and PAS.
In Nephrogenic
metaplasia, small glands show renal tubular
expression close to the urothelium. It is
associated with prior urothelial trauma due to
instrumentation, urethral catheterization,
infection, or calculi. It also occurs after renal
transplantation.
It is associated
with acute and chronic inflammation. Lesions are
composed of small tubules showing a variety of
histologic appearances. They may be small or
dilated and lined by a single layer of epithelial
cells that are cuboidal or flattened. Cells may
also show a hobnail or signet ring appearance.
When it involves the surface, it gives a papillary
appearance. The cells contain eosinophilic to
clear cytoplasm. The tubules may contain dense
eosinophilic material, resembling the thyroid.
Nephrogenic metaplasia can mimic prostate acinar
adenocarcinoma. A thickened eosinophilic hyaline
rim may form around the tubules, which helps
differentiate it from carcinoma. Carcinoma also
has prominent cellular atypia with a diffused and
disordered growth pattern. The cells are
amphophilic and pleomorphic, with large and
prominent nucleoli. Inflammation is rare.
Paneth cell-like
change can be seen in both benign and malignant
lesions. The presence of collections of prostatic
cells with eosinophilic granules in the cytoplasm
resembles intestinal Paneth cells.[34] Represents
either (a) PAS-positive, diastase-resistant
eosinophilic cytoplasmic granular change in the
benign prostatic epithelium or (b) endocrine
differentiation with neuroendocrine granules in
the dysplastic and malignant prostatic
epithelium.[35] There is no cytological atypia. It
is also called eosinophilic metaplasia.
It is a reactive
change of the prostatic epithelium to radiation
therapy, granulomatous prostatitis, and hormone
therapy. It is almost always associated with
inflammation. It can mimic prostatic
adenocarcinoma with Paneth cell differentiation.
Glands in adenocarcinoma are infiltrative and
angulated, often with collapsed lumina. However,
they tend to be of low grade and low stage.[36]
Paneth cell metaplasia has round, uniform, and
circumscribed groups of glands without atypia. Figure
2 shows the morphology of squamous
metaplasia, mucinous metaplasia, transitional cell
metaplasia, and paneth cell-like change.

|
| Figure
2: (a) Photomicrograph of
squamous metaplasia of prostate glands.
(100x, H&E). (b) Photomicrograph showing
mucinous metaplasia of prostate glands.
(400x, H&E). (c) Photomicrograph of
transitional cell metaplasia of prostate
glands showing multilayered epithelial
lining comprising cells with nuclear
grooves. (400x, H&E). (d)
Photomicrograph of Paneth cell-like change
showing eosinophilic granules in the
cytoplasm of glandular epithelial cells.
(400x, H&E). |
There are four types
of atrophy found in our study. They are simple,
partial, sclerotic, and postatrophic hyperplasia
(hyperplastic). Out of these, the most common
atrophy was simple atrophy, which is similar to
the study done by Postma et al., where
the incidence of simple atrophy was 91%.[37]
Atrophy is when
there is a decreased volume of cytoplasm in
prostatic acinar luminal cells. The decrease in
cytoplasm leads to an increased
nuclear-to-cytoplasmic ratio. This alteration is a
response to injury caused by chronic ischemia.[38]
Ischemia can be due to locoregional
arteriosclerosis which is common in the older male
population. Atrophy is also caused by radiation
treatment or androgen ablation.[38]
Simple atrophy is
when the decrease in cytoplasm is severe, making
the glandular epithelial cells appear flattened
and basophilic. The glands appear dilated. The
cell membrane lies just adjacent to the nucleus,
yielding a high nuclear-to-cytoplasmic ratio, and
is often associated with inflammation. Cystic
glandular dilation may also occur. In the article
by Trpkov et al., it is mentioned that
simple atrophy is the most common morphologic
variant, and the term ‘atrophy’ is typically used
to designate simple atrophy.[2] It is typically
associated with inflammation.[2] Chronic
inflammatory infiltrates are often found within
and around the foci of atrophy.[2] On the
contrary, inflammation is rarely seen in the foci
of prostatic adenocarcinoma.[2] Simple atrophy
occurs following treatment with antiandrogens and
radiation.[2]
Partial atrophy
occurs when the decrease in cytoplasm is moderate,
with cells having some pale eosinophilic
cytoplasm. The glands may have an infiltrative
appearance. Various luminal shapes can be seen.
They are infoldings, undulations, and straight
luminal borders. Since basal cells are difficult
to identify and due to prominent acinar
architectural distortion, partial atrophy can be
misinterpreted as low-grade prostatic
adenocarcinoma. This variant of atrophy is the
most problematic since it can appear disorganized
and infiltrative with irregular growth.[39] It
also lacks the basophilic appearance that is
typically seen in simple atrophy and postatrophic
hyperplasia.[39] Acinar adenocarcinoma has
prominent atypia in addition to diffused and
disordered growth.[39] Cells have amphophilic
cytoplasm and nuclear crowding. Nuclei are large
and atypical, comprising large prominent
nucleoli.[39] In carcinoma, blue-tinged mucin is
commonly seen, while corpora amylacea is rare.[39]
In Sclerotic
atrophy, there is marked sclerosis around the
acini. This makes the glands appear angular,
distorted, and infiltrative. Postatrophic
hyperplasia (hyperplastic atrophy) has a central
dilated duct with smaller atrophic acini around it
in a lobular configuration.[40] Stroma appears
sclerotic.
Prostatic
adenocarcinoma (atrophic type) can mimic simple
and sclerotic atrophy.[40] Atrophic adenocarcinoma
is often intermixed with non-atrophic conventional
prostate carcinoma and has less basophilic
cytoplasm and cytological atypia. Figure 3
shows the morphology of nephrogenic metaplasia,
partial atrophy, sclerotic atrophy, and
postatrophic hyperplasia.

|
| Figure
3: (a) Photomicrograph of
nephrogenic metaplasia where prostate
glands display a single cell lining with
fine chromatin and hobnailing (arrows)
(400x, H&E). (b) Photomicrograph of
partial atrophy in which many prostatic
acini are infiltrative and display
infoldings and angulated luminal contours
(100x, H&E). (c) Photomicrograph of
sclerotic atrophy showing marked sclerosis
surrounding prostatic acini (100x,
H&E). (d) Photomicrograph of
postatrophic hyperplasia showing a central
atrophic dilated duct with surrounding
smaller atrophic acini. (400x, H&E).
|
Conclusion
With a wide array of
benign lesions and mimics, it is important to be
aware of and get familiarised with the
characteristic morphological features of each
lesion, to identify them correctly and prevent any
false positive interpretation. In addition, proper
handling and processing of the tissue specimens
must be ensured. Special stains and immunostains
must be used whenever required.
References
- Egevad L, Delahunt B, Furusato B, Tsuzuki T,
Yaxley J, Samaratunga H. Benign mimics of
prostate cancer. Pathology. 2021
Jan;53(1):26-35
- Trpkov K. Benign mimics of prostatic
adenocarcinoma. Mod Pathol 2018;31
(Suppl 1):22–46.
- McNeal JE. Origin and evolution of benign
prostatic enlargement. Invest Urol. 1978;15(4):340–345
- Nwafor CC, Keshinro OS, Abudu EK. A
histopathological study of prostate lesions in
Lagos, Nigeria: A private practice experience. Niger
Med J 2015;56:338-43
- Dabir PD, Ottosen P, Hoyer S, Hamilton-Dutoit
S. Comparative analysis of three- and
two-antibody cocktails to AMACR and basal cell
markers for the immunohistochemical diagnosis of
prostate carcinoma. Diagn Pathol.
2012;7:81
- ICMR. Consolidated report of population based
cancer registries 2001-2004: incidence and
distribution of cancer. Bangalore (IND):
Coordinating Unit, National Cancer Registry
Programme, Indian Council of Medical Research;
2006
- Lakhtakia R, Bharadwaj R, Kumar VK, Mandal P,
Nema SK. Immunophenotypic characterization of
benign and malignant prostatic lesions. Med
J Armed Force India. 2007;63:243-8
- Garg M, Kaur G, Malhotra V, Garg R.
Histopathological spectrum of 364 prostatic
specimens including immunohistochemistry with
special reference to grey zone lesions. Prostate
Int. 2013;1(4):146-151
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 2:68
- Albasri A, El-Siddig A, Hussainy A, Mahrous M,
Alhosaini AA, Alhujaily A. Histopathologic
characterization of prostate diseases in
Madinah, Saudi Arabia. Asian Pac J Cancer
Prev. 2014;15(10):4175-9
- Pushpa N, Goyal R, Bhamu S. Histopathological
Spectrum of Prostatic Lesions and Their
Correlation with Serum Prostate Specific Antigen
Levels. Asian Journal of Pharmaceutical and
Clinical Research. Feb. 2024;17(2):36-39.
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:5
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:4
- MacLennan GT, Resnik MI, Bostwick DG.
Pathology for Urologists. Pennsylvania.
Saunders; 2003; 3:84
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:8
- Warrick J, Humphrey PA. Nonspecific
granulomatous prostatitis. J Urol. 2012
Jun;187(6):2209-10
- Furusato B, Koff S, McLeod DG, Sesterhenn IA.
Sarcoidosis of the prostate. J Clin Pathol.
2007 Mar;60(3):325-6
- Epstein JI, Hutchins GM. Granulomatous
prostatitis: distinction among allergic,
nonspecific, and post-transurethral resection
lesions. Hum Pathol. 1984
Sep;15(9):818-25.
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:10
- Mahapatra QS, Mohanty P, Nanda A, Mohanty L.
Histomorphological study of prostatic
adenocarcinoma and its mimics. Indian J
Pathol Microbiol. 2019
Apr-Jun;62(2):251-260
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:32
- Thorson P, Swanson PE, Vollmer RT, Humphrey
PA. Basal cell hyperplasia in the peripheral
zone of the prostate. Mod Pathol. 2003
Jun;16(6):598-606
- Kruithof-Dekker IG, Têtu B, Janssen PJ, Van
der Kwast TH. Elevated estrogen receptor
expression in human prostatic stromal cells by
androgen ablation therapy. J Urol. 1996
Sep;156(3):1194-7
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:26
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:36
- Bostwick DG, Qian J. High-grade prostatic
intraepithelial neoplasia. Mod Pathol.
2004 Mar;17(3):360-79
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 2:68
- Kumar V, Abbas AK, Aster JC. Robbins Basic
Pathology. 10th ed. Kumar V, Abbas AK, Aster JC,
editors. Philadelphia, PA: Elsevier - Health
Sciences Division; 2021
- Abdollahi A, Ayati M. Frequency and outcome of
metaplasia in needle biopsies of prostate and
its relation with clinical findings. Urol J.
2009 Spring;6(2):109-13
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:38
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:40
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:41
- Zhou M, Jiang Z, Epstein JI. Expression and
diagnostic utility of
alpha-methylacyl-CoA-racemase (P504S) in foamy
gland and pseudohyperplastic prostate cancer. Am
J Surg Pathol. 2003 Jun;27(6):772-8
- Weaver MG, Abdul-Karim FW, Srigley JR. Paneth
cell-like change and small cell carcinoma of the
prostate. Two divergent forms of prostatic
neuroendocrine differentiation. Am J Surg
Pathol. 1992 Oct;16(10):1013-6
- Weaver MG, Abdul-Karim FW, Srigley J, Bostwick
DG, Ro JY, Ayala AG. Paneth cell-like change of
the prostate gland. A histological,
immunohistochemical, and electron microscopic
study. Am J Surg Pathol. 1992
Jan;16(1):62-8
- Salles DC, Mata DA, Epstein JI. Significance
of Paneth cell-like differentiation in prostatic
adenocarcinoma: a retrospective cohort study of
80 cases. Hum Pathol. 2020 Aug;102:7-12
- Postma R, Schröder FH, van der Kwast TH.
Atrophy in prostate needle biopsy cores and its
relationship to prostate cancer incidence in
screened men. Urology. 2005
Apr;65(4):745-9
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:12
- Xu Y, Wang Y, Zhou R, Li H, Cheng H, Wang Z,
Zhang J. The benign mimickers of prostatic
acinar adenocarcinoma. Chin J Cancer Res. 2016
Feb;28(1):72-9
- Zynger DL, Parwani AV, Suster S. Prostate
Pathology. New York. Demos Medical; 2015; 1:22
|



















