|
Introduction
Blood
stream infection (BSI) is a leading cause of
mortality and morbidity worldwide. The pathogen in
the blood, quickly spreads to various organs in
the body, resulting in septic shock and organ
dysfunction. This makes it a formidable medical
emergency, requiring prompt diagnosis and
treatment. The diagnosis and management of the
BSIs can be very challenging, as it requires high
degree of suspicion, prompt investigations in the
form of blood cultures and other laboratory
parameters and immediate administration of
appropriate empirical antibiotics followed by
continuous assessment with escalation or
de-escalation of the antibiotics depending upon
the patient’s clinical response and culture
reports. The prognosis of the patient also depends
on finding the source of the infection and
appropriate source control of the infection focus.
(1) As a result, the overall crude mortality rates
of BSIs are high and may vary between 15% and 30%.
BSIs also have considerable effect on the
admission to intensive care units (ICUs), length
of hospital stay and cost of health care. The
problem gets further complicated by antibiotic
resistance. This makes bloodstream infection a
very important burden to the health care systems.
(2)
The burden of BSIs
in the Lower- and Middle-income countries (LMIC)
is higher than the developed countries, especially
due to higher proportions of antimicrobial
resistance (AMR). Unfortunately, there is a
scarcity of studies which describe the
epidemiology and outcomes of the patients with
blood stream infections from these regions. (2)
There is an urgent need for the knowledge on the
causative agents and outcomes of the blood stream
infections from different parts of India. It is
also equally important to monitor the antibiotic
resistance of the pathogens causing BSIs and their
epidemiology. This will ensure improvement of the
health care systems and thus better healthcare
service will be provided to community. (3) Gram
negative bacterial pathogens are shown to be
common cause of blood stream infections, with high
level of resistance against the common empiric
antibiotics. (4–7) In this study we try to fill
the gaps in the knowledge and present the
epidemiology of the Gram-negative bacterial blood
stream infection (GNBSI), prevalence of carbapenem
resistance, patient management and outcome in an
Indian tertiary care hospital.
Methodology
This retrospective
study was conducted, after obtaining the ethical
clearance from the institute ethics committee.
(FMMC/FMIEC/455/2022) The patients ≥ 18 years of
age, with Gram negative bacteria (GNB) growth in
blood culture, during the study period August 2020
to May 2022 were included in the study. The
selection of the patients for the study is
described in the Figure 1.

|
| Figure
1: Flowchart of the study population |
Only monomicrobial
Gram negative bacterial blood stream infections
(GNBSI) were included. Patients with ≥2 bacteria
in blood were excluded from the study. Patients
with ≥2 episodes of BSIs with different bacteria
were also excluded from the study.
Definitions
GNBSI was defined as
at least one blood culture positive for growth of
GNB and any one or more of following features of
blood stream infections like fever, hypothermia,
tachypnoea, altered mental status, multiple organ
dysfunction syndrome, total leucocyte count
>11.0 ×109/L. Source of the GNBSI
was determined microbiologically by the documented
isolation of the same GNB from another site of
infection by culture of the corresponding
sample.(8) The electronic case records were
screened for the presence of co-morbidities. The
electronic case records were evaluated for the
documented administration of antibiotics,
ceftriaxone, piperacillin-tazobactam, meropenem,
tigecycline and polymyxin B during the hospital
stay. The event of intensive care unit (ICU)
admission, central venous catheter (CVC)
insertion, mechanical ventilation and vasopressor
administration anytime during the hospital stay
were noted. The crude in hospital mortality was
noted from the electronic case records. The death
at 7 days or 30 days was calculated from the day
of collection of the positive blood culture
sample. (2)
Microbiology
BACTECTM
aerobic system (Becton Dickinson, Maryland, USA)
was used to process blood cultures. The positive
blood cultures were processed further by Gram
stain and subcultures on 5% sheep blood agar
(HiMedia Laboratories Pvt. Ltd, Mumbai, India) and
MacConkey agar (HiMedia Laboratories Pvt. Ltd,
Mumbai, India). The growth was identified by the
standard biochemical methods and appropriate
antibiotic susceptibility testing was done by
modified Kirby Bauer method, using the appropriate
antibiotic discs (HiMedia Laboratories Pvt. Ltd,
Mumbai, India). Reporting of susceptibility was
done according to the Clinical Laboratory
Standards Institute (CLSI) guidelines.(9)
Ampicillin 10 µg, amoxicillin clavulanic acid
20/10 µg, cefuroxime 30 µg, ceftriaxone 30 µg,
cefotaxime 30 µg, piperacillin tazobactam 100/10
µg, imipenem 10 µg, meropenem 10 µg, amikacin 30
µg, co-trimoxazole 1.25/23.75 µg and ciprofloxacin
5µg discs were used for antibiotic susceptibility
testing. A GNB was considered to be carbapenem
resistant if it was resistant to either meropenem
or imipenem, on antibiotic susceptibility testing.
The analysis of antibiogram was done using WHONET
software.
Statistical
analysis
The data was
processed for statistical analysis using the IBM
SPSS Statistics (version 23). The continuous
variables were checked for normality of
distribution for each subset. The continuous
variables with normal distribution were expressed
as mean with standard deviation (SD), whereas the
continuous variables with non-normal distribution
were expressed as median with interquartile range
(IQR). The categorical variables were expressed as
absolute counts and frequency percentages.
A comparison was
made between the carbapenem susceptible and
carbapenem resistant GNBSI by Chi square test,
Fisher’s Exact test, Student t test or
Mann-Whitney U test, as applicable
according to the type of variable. (table 1)
The survival
analysis was performed using the in-hospital crude
mortality rates at days 7 and 30, from the day of
collecting the blood culture sample positive for
the GNB. The length of stay after the positive
GNBSI was used for the calculation of hazard ratio
(HR), 95% confidence interval by the univariate
analysis of the possible independent variables by
Cox proportional hazards model. The variables with
P values < 0.1 for HR, during the univariate
analysis were used for calculating the adjusted
hazard ratio (aHR) with 95% confidence interval
(CI) by multivariate survival analysis.
A similar mortality
analysis at days 7 and 30 was performed among the
subset of patients who had an event of ICU
admission during the hospital stay.
Results
During the study
period of 22 months, from August 2020 to May 2022,
monomicrobial GNBSIs were detected in 375
patients. Among the patients, 204 (54.4%) were
males, median age was 60 (IQR 48-69) years. The
GNBSIs were caused commonly by Escherichia
coli, 105 (28.0%), Klebsiella
pneumoniae, 100 (26.7%) and Acinetobacter
species, 65 (17.3%), as shown in figure 2.

|
Figure 2: Bacterial
epidemiology of monomicrobial GNBSIs
(n=375)
Abbreviations: GNBSI, Gram negative
bacterial blood stream infections |
As shown in Figure
3, Acinetobacter species was the most
resistant common pathogen, followed by Klebsiella
pneumoniae compared to Escherichia
coli.
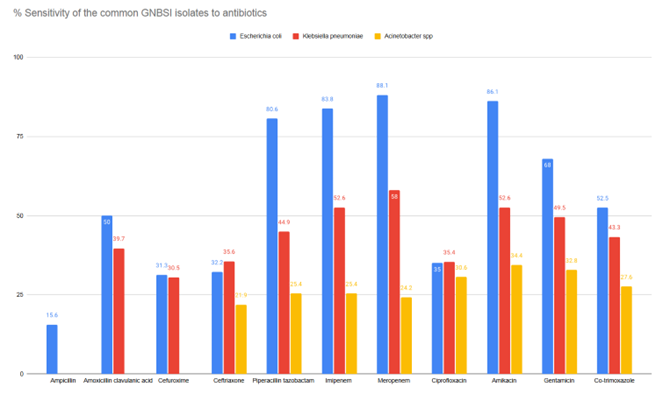
|
| Figure
3: Antibiogram of the common pathogens
causing GNBSIs |
The characteristics
of carbapenem susceptible and carbapenem resistant
Gram-negative bacterial blood stream infections
(CR-GNBSI) are shown in Table 1. The total
duration of hospital stay was longer among
patients with CR-GNBSIs (p value, 0.000). The
length of hospital stay after the positive blood
culture was not different between the 2 groups.
CR-GNBSIs were associated with COVID-19 infection
(p value, 0.000) and use of immunosuppressive
drugs (p value, 0.005). CR-GNBSIs were associated
more with the lung infections as source (p value,
0.000). The CR-GNBSIs were associated with
associated with administration of the higher end
antibiotics like meropenem, tigecycline and
polymyxin B (p value, 0.000). They were also
associated with the insertion of central venous
catheter, mechanical ventilation, vasopressor
administration and ICU admission (p value, 0.000).
There was an association between mortality and the
CR-GNBSIs (p value, 0.000).
|
Table 1: Comparison of patient
characteristics according to the
carbapenem resistance of GNBSIs
|
|
Total GNBSIs [N=375 (100%)]
|
Carbapenem susceptible GNBSIs
N=238 (63.47%)
|
Carbapenem resistant GNBSIs N=137
(36.53%)
|
P value
|
|
Male
|
204 (54.4%)
|
129 (54.2%)
|
75 (54.7%)
|
0.919†
|
|
Median Age (interquartile range) in years
|
60 (48-69)
|
60 (48-68)
|
60 (46-70)
|
0.185*
|
|
Total length of hospital stay (median,
IQR) in days
|
11 (6-18)
|
9 (4-16)
|
14 (9-24.50)
|
0.000*
|
|
Fever
|
255 (68.0%)
|
168 (70.6%)
|
87 (63.5%)
|
0.157†
|
|
Diabetes mellitus
|
201 (53.6%)
|
135 (56.7%)
|
66 (48.2%)
|
0.110†
|
|
Hypertension
|
190 (50.7%)
|
114 (47.9%)
|
76 (55.5%)
|
0.158†
|
|
Ischemic heart disease
|
58 (15.5%)
|
33 (13.9%)
|
25 (18.2%)
|
0.258†
|
|
Chronic liver disease
|
37 (9.9%)
|
26 (10.9%)
|
11 (8.0%)
|
0.365†
|
|
COVID-19 infection
|
66 (17.6%)
|
25 (10.5%)
|
41 (29.9%)
|
0.000†
|
|
Chronic kidney disease
|
85 (22.7%)
|
54 (22.7%)
|
31 (22.6%)
|
0.989†
|
|
Obstructive airway disease
|
20 (5.3%)
|
10 (4.2%)
|
10 (7.3%)
|
0.199†
|
|
Immunosuppressive drugs
|
110 (29.3%)
|
58 (24.4%)
|
52 (38.0%)
|
0.005†
|
|
Malignancy
|
69 (18.4%)
|
49 (20.6%)
|
20 (14.6%)
|
0.149†
|
|
Source
|
0.000†
|
|
Urinary tract
|
64 (17.1%)
|
54 (22.7%)
|
10 (7.3%)
|
|
|
Pulmonary
|
33 (8.8%)
|
9 (3.8%)
|
24 (17.5%)
|
|
|
Skin and soft tissue
|
8 (2.1%)
|
4 (1.7%)
|
4 (2.9%)
|
|
|
Abdomen
|
8 (2.1%)
|
4 (1.7%)
|
4 (2.9%)
|
|
|
Unknown and other sources
|
262 (69.9%)
|
167 (70.2%)
|
95 (69.3%)
|
|
|
Microbiology
|
0.000†
|
|
Escherichia coli
|
105 (28.0%)
|
98 (37.8%)
|
15 (10.9%)
|
|
|
Klebsiella pneumoniae
|
100 (26.7%)
|
52 (21.8%)
|
48 (35.0%)
|
|
|
Acinetobacter species
|
65 (17.3%)
|
17 (7.1%)
|
48 (35.0%)
|
|
|
Pseudomonas aeruginosa
|
17 (4.5%)
|
13 (5.5%)
|
4 (2.9%)
|
|
|
Other Enterobacterales
|
43 (11.5%)
|
37 (15.5%)
|
6 (4.4%)
|
|
|
Other Non-fermenting Gram negative
bacilli
|
40 (10.7%)
|
24 (10.1%)
|
16 (11.7%)
|
|
|
Other GNB
|
5 (1.3%)
|
5 (2.1%)
|
0
|
|
|
Ceftriaxone administered
|
76 (20.3%)
|
45 (18.9%)
|
31 (22.6%)
|
0.388†
|
|
Piperacillin tazobactam administered
|
183 (48.8%)
|
122 (51.3%)
|
61 (44.5%)
|
0.209†
|
|
Meropenem administered
|
204 (54.4%)
|
112 (47.1%)
|
92 (67.2%)
|
0.000†
|
|
Tigecycline administered
|
36 (9.6%)
|
10 (4.2%0)
|
26 (19.0%)
|
0.000†
|
|
Polymyxin B administered
|
50 (13.3%)
|
13 (5.5%)
|
37 (27.0%)
|
0.000†
|
|
Central venous catheter insertion
|
119 (68.3%)
|
57 (23.9%)
|
62 (45.3%)
|
0.000†
|
|
Mechanical ventilation
|
119 (68.3%)
|
44 (18.5%)
|
75 (54.7%)
|
0.000†
|
|
Vasopressor administered
|
146 (38.9%)
|
77 (32.4%)
|
69 (50.4%)
|
0.001†
|
|
ICU admission
|
232 (61.9%)
|
125 (52.5%)
|
107 (78.1%)
|
0.000†
|
|
7-day mortality
|
127 (33.9%)
|
66 (27.7%)
|
61 (44.5%)
|
0.001†
|
|
30-day mortality
|
151 (40.3%)
|
76 (31.9%)
|
75 (54.7%)
|
0.000†
|
|
Overall mortality
|
153 (40.8%)
|
77 (32.4%)
|
76 (55.5%)
|
0.000†
|
|
* - Mann Whitney U test; †-
Chi square test;
Abbreviations: GNBSI, Gram negative
bacterial blood stream infection; IQR,
Interquartile range; GNB, Gram negative
bacteria; ICU, Intensive care unit
|
Mortality
analyses
Overall, in-hospital
crude mortality among the patients with
monomicrobial GNBSI in our study was 40.3%.
Mortality at 7 days and 30 days were 33.9% and
40.3% respectively. As shown in table 2, ICU
admission, vasopressor use, immunosuppressive drug
administration were independent risk factors for
mortality.
|
Table 2: Multivariate analysis
for mortality among patients with
monomicrobial GNBSIs (n=375)
|
|
7-day mortality
|
30-day mortality
|
|
aHR
|
P
|
95% CI
|
aHR
|
P
|
95% CI
|
|
Total Length of Hospital Stay
|
0.867
|
0.000
|
0.836-0.899
|
0.871
|
0.000
|
0.844-0.899
|
|
Age
|
|
1.006
|
0.270
|
0.995-1.018
|
|
Fever
|
0.774
|
0.176
|
0.534-1.122
|
0.763
|
0.124
|
0.541-1.077
|
|
Chronic liver disease
|
1.225
|
0.433
|
0.738-2.033
|
1.238
|
0.395
|
0.757-2.026
|
|
Covid-19 infection
|
0.987
|
0.959
|
0.585-1.663
|
0.932
|
0.777
|
0.573-1.516
|
|
Immunosuppressive drugs
|
1.585
|
0.059
|
0.982-2.557
|
1.706
|
0.019
|
1.092-2.665
|
|
Source
|
|
0.801
|
|
|
0.819
|
|
|
Urinary tract
|
0.955
|
0.894
|
0481-1.896
|
0.941
|
0.858
|
0.485-1.827
|
|
Lungs
|
1.325
|
0.359
|
0.727-2.415
|
1.333
|
0.303
|
0.771-2.306
|
|
Skin and soft tissue
|
1.054
|
0.945
|
0.234-4.751
|
1.069
|
0.917
|
0.305-3.751
|
|
Abdomen
|
1.817
|
0.368
|
0.495-6.669
|
1.536
|
0.497
|
0.445-5.300
|
|
Unknown
|
Ref (1)
|
Ref (1)
|
|
Microbiology
|
|
0.550
|
|
|
0.666
|
|
|
Escherichia coli
|
1.091
|
0.854
|
0.433-2.745
|
0.799
|
0.594
|
0.351-1.821
|
|
Klebsiella pneumoniae
|
2.060
|
0.123
|
0.823-5.161
|
1.363
|
0.459
|
0.600-3.100
|
|
Acinetobacter species
|
1.080
|
0.914
|
0.268-4.350
|
1.707
|
1.128
|
0.597-4.878
|
|
Pseudomonas aeruginosa
|
1.138
|
0.832
|
0.344-3.768
|
1.128
|
0.810
|
0.422-3.013
|
|
Other Enterobacterales
|
1.453
|
0.475
|
0521-4.051
|
1.085
|
0.855
|
0.451-2.612
|
|
Other GNB
|
1.202
|
0.828
|
0.228-6.347
|
0.864
|
0.858
|
0.175-4.273
|
|
Other Non-fermenting GNB
|
Ref (1)
|
Ref (1)
|
|
Carbapenem resistance
|
|
0.294
|
|
|
0.402
|
|
|
CRA-GNBSI
|
3.524
|
0.040
|
1.058-11.738
|
1.315
|
0.546
|
0.540-3.204
|
|
CRKP – GNBSI
|
1.277
|
0.524
|
0.601-2.714
|
1.372
|
0.387
|
0.670-2.808
|
|
Other CRE – GNBSI
|
1.408
|
0.489
|
0.535-3.709
|
1.916
|
0.095
|
0.893-4.109
|
|
Other CR-GNBSI
|
1.018
|
0.977
|
0.296-3.497
|
0.803
|
0.668
|
0.295-2.187
|
|
Carbapenem susceptible GNB
|
Ref (1)
|
Ref (1)
|
|
Meropenem administration
|
1.062
|
0.793
|
0.678-1.663
|
0.998
|
0.994
|
0.661-1.508
|
|
Central venous catheter insertion
|
0.621
|
0.032
|
0.401-0.961
|
|
|
Mechanical ventilation
|
1.093
|
0.686
|
0.709-1.686
|
1.241
|
0.304
|
0.822-1.874
|
|
Vasopressor administration
|
2.496
|
0.000
|
1.495-4.167
|
2.569
|
0.000
|
1.612-4.093
|
|
ICU admission
|
6.714
|
0.000
|
2.824-15.961
|
8.300
|
0.000
|
3.383-20.362
|
|
P<0.05 was considered statistically
significant
Abbreviations: aHR, adjusted Hazard Ratio;
CI, Confidence interval; GNBSI, Gram
negative bacterial blood stream infection;
GNB, Gram negative bacteria; ICU,
Intensive care unit; CRA, carbapenem
resistant Acinetobacter species;
CRKP, carbapenem resistant Klebsiella
pneumoniae; CRE, carbapenem
resistant Enterobacterales; CR-GNBSI,
carbapenem resistant Gram-negative
bacterial blood stream infection
|
Table 3 describes a
sub analysis for mortality, among the 232 patients
who had event of ICU admission during hospital
stay. When compared to patients with carbapenem
susceptible-GNBSI (CS-GNBSI), carbapenem resistant
Acinetobacter species- GNBSI (CRA-GNBSI)
and carbapenem resistant Klebsiella
pneumoniae GNBSI (CRKP-GNBSI) were
independent risk factors for mortality at 7 days
and 30 days. Vasopressor administration was an
independent risk factor for mortality at both 7
days and 30 days.
|
Table 3: Multivariate analysis
for mortality among patients with an
event of ICU admission during hospital
stay (n=232)
|
|
7-day mortality
|
30-day mortality
|
|
aHR
|
P
|
95% CI
|
aHR
|
P
|
95% CI
|
|
Total length of hospital stay
|
0.872
|
0.000
|
0.841-0.904
|
0.880
|
0.000
|
0.853-0.907
|
|
Fever
|
|
0.898
|
0.540
|
0.638-1.266
|
|
Diabetes mellitus
|
|
|
|
0.787
|
0.186
|
0.551-1.123
|
|
Hypertension
|
0.716
|
0.075
|
0.496-1.034
|
0.768
|
0.151
|
0.535-1.101
|
|
Chronic liver disease
|
1.020
|
0.940
|
0.612-1.698
|
0.975
|
0.921
|
0.591-1.609
|
|
Carbapenem resistance
|
|
0.000
|
|
|
0.001
|
|
|
Carbapenem susceptible GNB
|
1 (Ref)
|
|
|
CRA - GNBSI
|
3.553
|
0.000
|
2.198-5.742
|
2.658
|
0.000
|
1.671-4.228
|
|
CRKP - GNBSI
|
2.665
|
0.001
|
1.524-4.662
|
2.241
|
0.003
|
1.324-3.791
|
|
Other CRE- GNBSI
|
1.280
|
0.571
|
0.544-3.015
|
1.664
|
0.153
|
0.827-3.347
|
|
Other CR-GNBSI
|
0.970
|
0.954
|
0.343-2.740
|
1.176
|
0.716
|
0.491-2.814
|
|
Central venous catheter insertion
|
0.615
|
0.026
|
0.401-0.945
|
0.689
|
0.055
|
0.471-1.008
|
|
Mechanical ventilation
|
|
1.384
|
0.103
|
0.937-2.045
|
|
Vasopressor administration
|
2.151
|
0.001
|
1.375-3.365
|
2.279
|
0.000
|
1.491-3.483
|
|
*P<0.05 was considered statistically
significant
Abbreviations: aHR, adjusted Hazard Ratio;
CI, Confidence interval; GNBSI, Gram
negative bacterial blood stream infection;
ICU, Intensive care unit; CRA – GNBSI
Carbapenem resistant Acinetobacter
species Gram negative bacterial blood
stream infection; CRKP – GNBSI, Carbapenem
resistant Klebsiella pneumoniae Gram
negative bacterial blood stream infection;
CRE- GNBSI, Carbapenem resistant
Enterobacterales Gram negative bacterial
blood stream infection; CR-GNBSI,
Carbapenem resistant Gram-negative
bacterial blood stream infection.
|
Discussion
Blood stream
infections are medical emergencies, requiring
immediate diagnosis and treatment and continuous
monitoring of the patient for the response to
treatment.
Escherichia coli
was the most common pathogen, followed by Klebsiella
pneumoniae and Acinetobacter
species in the present study. Segala et al, in a
study in Italy, also reported Escherichia
coli, as the most common GNB pathogen,
followed by Klebsiella pneumoniae. (10)
Klebsiella pneumoniae and Acinetobacter
baumanii were the most common pathogens
that were resistant to carbapenems, in the current
study. Similarly, in a study at Delhi, by P et al,
the most common difficult to treat GNBSI were due
to Klebsiella pneumoniae and Acinetobacter
baumanii. (11)
We recorded in
hospital crude mortality of 40.8% among the
patients with GNBSI during the study period.
Similar mortality rates have been reported in many
studies all over the world and India, during the
period of COVID-19 pandemic. Unterberg et al,
reported an increase in hospital crude mortality
rate from 33% to 52% among the patients with
sepsis during the pandemic period. He demonstrated
a high initial sequential organ failure assessment
(SOFA) score at admission among the patients
during the pandemic, indicating delayed
presentations of the patients to the hospital.
(12) In an Indian study, during the same time, P
et al, recorded an overall mortality rate of 50.8%
among the patients with GNBSI episodes. (11) This
high mortality could be due to late presentation
of patient to the hospital, in critical stage with
comorbidities and infection with multidrug
resistant (MDR) bacteria leading to failure of
treatment.
On multivariate
mortality analysis, we found that ICU admission
was the strongest independent risk factor
associated with mortality at both 7 days
(aHR,6.714, p value, 0.000, 95% CI, 2.824-15.961)
and 30 days (aHR, 8.300, p value- 0.000, 95%
CI,3.383-20.362). Use of vasopressors was also
independent risk factor for the mortality at days
7(aHR, 2.496, p value, 0.001, 95% CI, 1.495-4.167)
and 30 (aHR, 2.569, p value, 0.000, 95%
CI,1.612-4.093), in all the patients (table 2) and
the subset of patients with event of ICU admission
(Table 3). These findings indicate that the
mortality was highest among the patients who were
critically ill. These findings are consistent with
similar studies, where the patients with critical
conditions in ICU, indicated by high Pitt score,
Charlson comorbidity index, SOFA score or APACHE
score at the time of BSI were largest risk factor
among patients with sepsis. (8,12–18)
We found that the
use of immunosuppressive drugs was associated with
higher mortality among the patients at 30 days
(aHR, 1.706, p value, 0.019, 95% CI, 1.092-2.665)
after the episode of GNBSI. Interestingly we found
that fever was present in only 68.0% of the
patients with episode of GNBSI. (Table 1) Also, it
was a protective factor against mortality, on
univariate analysis. (Supplementary Table 1) The
protective effect of fever in patients with sepsis
has been documented by Rumbus et al and others.
(19,20) The decreased rate of fever in GNBSIs may
be due to the increased administration of the
immunosuppressive drugs during the pandemic, which
led to reduced fever and inflammatory markers,
making it difficult to objectively assess the
response to therapy, as reported by Segala et
al.(10) This is also demonstrated in our study,
where we find that Covid 19 infection was a risk
factor for mortality on univariate analysis, but
it was not an independent risk factor, during
multivariate analysis. (Table 2 and supplementary
Table 1). Palanisamy et al, reported 64 patients
with Covid-19 infections in ICU who developed BSI.
All the patients were on corticosteroids and
succumbed to the BSI. (17)
CR-GNBSI was
detected in 36.53% of the patients, in the present
study. There are few studies from India,
presenting the epidemiology of carbapenem
resistance in GNBSIs. In one such study during the
similar period, Naveenraj P et al reported 111/240
cases of GNBSIs to be caused by carbapenem
resistant bacteria. (11) Kaur et al, in a
multicentric longitudinal study from 2017 to 2022,
reports that there has been significant increase
in resistance to carbapenem resistance among cases
of GNBSIs.(21) We find in our study that
carbapenem resistance was not an independent risk
factor for mortality, among all the patients
during the study period (Table 2), we found that,
among the subset of patients with ICU admission,
CRA-GNBSI and CRKP GNBSIs were independent risk
factors for mortality at 7 and 30 days, when,
compared to patients with CS-GNBSIs.(Table 2)
Palanisamy et al also has reported Acinetobacter
baumannii as the most common GNB, followed
by Klebsiella pneumoniae and
non-fermenters causing BSIs among the COVID-19
patients in ICU. (17) Most of the countries
worldwide have reported increased hospital
acquired infections and AMR during the pandemic,
due to the unprecedented workload, beyond the
capacity of the healthcare and laboratory systems.
There was a breakdown of HAI surveillance and
breach in the infection control practices.
(10,11,15,17,22–29)
It is important to
note that in 65.9% of GNBSI cases in our study,
microbiological confirmation of the source was not
available. Though microbiological source
determination may not be possible in every case of
GNBSI, it would certainly help to effectively
treat the infection and reduce the AMR rates.
(1,2,8) The culture utilization rates need to be
studied in context of empirical antimicrobial
prescription and development of AMR. Lim et al, in
a study, recommends including blood culture
utilization rates in addition to AMR surveillance
reports. (30) There is a lack of studies regarding
this from India. Also, there is a risk of
overestimating the severity of BSIs and
over-reporting of MDR-BSIs, if the blood cultures
are not prescribed timely. The lack of culture
testing may also promote AMR due to prolonged use
of inappropriate empirical antibiotics. The cost
and mortality differences need to be studied with
respect to the utility of microbiological cultures
for infections. (2)
This study has
relatively large sample size of 375 consecutive
monomicrobial GNBSIs among the patients admitted
to the hospital. The study provides valuable
insights into the problems associated with GNBSIs,
especially during the pandemic. The study
identifies the risk of immunosuppressive drugs
associated with mortality.
The study has some
limitations. The study is retrospective and may be
influenced by the errors of data documentation. We
were not able to collect the data on source
control and appropriateness of empirical
antibiotic therapy, which may influence the
mortality among the patients. Since the study was
conducted in a single hospital, it may not fully
reflect the situation in other healthcare
settings. The blood cultures were sent according
to the discretion of the clinicians, which if
inadequate, may have resulted in overestimation of
severe GNBSIs and the rates of CR-GNBSIs.
Conclusion
Our study explores
the epidemiology and clinical outcomes of 375
monomicrobial GNBSIs among the patients admitted
in an Indian tertiary care hospital from August
2020 to May 2022. Escherichia coli, Klebsiella
pneumoniae and Acinetobacter
species were the most common bacteria causing
GNBSIs. Carbapenem resistance was observed in
36.56% of the isolates. Overall mortality was
40.8% among the patients with GNBSIs. Events of
ICU admission and vasopressor administration were
independent risk factors for death. Use of
immunosuppressive drugs was an independent risk
factor for mortality. Among the patients with ICU
admission, carbapenem resistant Acinetobacter
species GNBSIs and carbapenem resistant Klebsiella
pneumoniae GNBSIs were independent risk
factors for mortality. Our study indicates that
there is a need for monitoring the carbapenem
resistance and measures need to be taken to reduce
it, as there are very few antibiotics available
for the treatment of carbapenem resistant
pathogens. Similarly, measures also need to be
taken for early identification and effective
treatment of GNBSIs to facilitate better outcomes.
Hence there is need to empower the antibiotic
stewardship practices in the healthcare systems.
References
- Aslan AT, Tabah A, Köylü B et al. Epidemiology
and risk factors of 28-day mortality of
hospital-acquired bloodstream infection in
Turkish intensive care units: a prospective
observational cohort study. J Antimicrob
Chemother. 2023;78(7):1757–68.
- Allel K, Stone J, Undurraga EA et al. The
impact of inpatient bloodstream infections
caused by antibiotic-resistant bacteria in low-
and middle-income countries: A systematic review
and meta-analysis. PLOS Med.
2003;20(6):e1004199.
- Zeng Q, Xiang B, Liu Z. Profile and Antibiotic
Pattern of Blood Stream Infections of Patients
Receiving Hematopoietic Stem Cell Transplants in
Southwest China. Infect Drug Resist
2022;15:2045–54.
- Singh AK, Venkatesh V, Singh RP, Singh M.
Bacterial and antimicrobial resistance profile
of bloodstream infections: A hospital-based
study. CHRISMED Journal of Health and
Research. 2014;1(3):140-4.
- Prabhash K, Medhekar A, Ghadyalpatil N et al.
Blood stream infections in cancer patients: A
single center experience of isolates and
sensitivity pattern. Indian J Cancer.
2010;47(2):184.
- Thacker N, Pereira N, Banavali S et al.
Epidemiology of blood stream infections in
pediatric patients at a Tertiary Care Cancer
Centre. Indian J Cancer. 2014;51(4):438.
- Wattal C, Raveendran R, Goel N, Oberoi JK, Rao
BK. Ecology of blood stream infection and
antibiotic resistance in intensive care unit at
a tertiary care hospital in North India. Braz
J Infect Dis. 2014;18(3):245–51.
- Santoro A, Franceschini E, Meschiari M et al.
Epidemiology and Risk Factors Associated With
Mortality in Consecutive Patients With Bacterial
Bloodstream Infection: Impact of MDR and XDR
Bacteria. Open Forum Infect Dis.
2020;7(11): ofaa461.
- CLSI. Performance Standards for Antimicrobial
Susceptibility Testing. 33rd ed.
- Segala FV, Pafundi PC, Masciocchi C et al.
Incidence of bloodstream infections due to
multidrug-resistant pathogens in ordinary wards
and intensive care units before and during the
COVID-19 pandemic: a real-life, retrospective
observational study. Infection. 2023;51(4):1061–9.
- Naveenraj P, Kumar D, Meena DS et al.
Difficult-to-treat resistant gram-negative blood
stream infections - the beginning of a superbug
era - a prospective observational study. Indian
J Med Microbiol. 2023;44:100364.
- Unterberg M, Rahmel T, Rump K et al. The
impact of the COVID-19 pandemic on non-COVID
induced sepsis survival. BMC Anesthesiol.
2022;22(1):12.
- Bass SN, Bauer SR, Neuner EA, Lam SW. Impact
of Combination Antimicrobial Therapy on
Mortality Risk for Critically Ill Patients with
Carbapenem-Resistant Bacteremia. Antimicrob
Agents Chemother. 2015;59(7):3748–53.
- Wang J, Zhang J, Wu Z-H et al. Clinical
Characteristics and Prognosis Analysis of
Acinetobacter baumannii Bloodstream Infection
Based on Propensity Matching. Infect Drug
Resist. 2022;15:6963–74.
- Mantzarlis K, Deskata K, Papaspyrou D et al.
Incidence and Risk Factors for Blood Stream
Infection in Mechanically Ventilated COVID-19
Patients. Antibiotics. 2022;11(8):1053.
- Vidaur L, Eguibar I, Olazabal A et al. Impact
of antimicrobial stewardship in organisms
causing nosocomial infection among COVID-19
critically ill adults. Eur J Intern Med. 2024;119:93–8.
- Palanisamy N, Vihari N, Meena DS, Kumar D,
Midha N, Tak V, et al. Clinical profile of
bloodstream infections in COVID-19 patients: a
retrospective cohort study. BMC Infect Dis.
2021;21(1):933.
- Lin XC, Li CL, Zhang SY, Yang XF, Jiang M. The
Global and Regional Prevalence of
Hospital-Acquired Carbapenem-Resistant
Klebsiella pneumoniae Infection: A Systematic
Review and Meta-analysis. Open Forum Infect
Dis. 2023;11(2):ofad649.
- Rumbus Z, Matics R, Hegyi P et al. Fever is
Associated with Reduced, Hypothermia with
Increased Mortality in Septic Patients: A
Meta-Analysis of Clinical Trials. PLoS One.
2017 Jan 12;12(1):e0170152.
- Shimazui T, Nakada T-A, Walley KR et al.
Significance of body temperature in elderly
patients with sepsis. Crit Care. 2020;24(1):387.
DOI:10.1186/s13054-020-02976-6
- Kaur J, Singh H, Sethi T. Emerging trends in
antimicrobial resistance in bloodstream
infections: multicentric longitudinal study in
India (2017–2022). Lancet Reg Health -
Southeast Asia. 2024 Jul;26:100412.
- Cataldo MA, Tetaj N, Selleri M et al.
Incidence of bacterial and fungal bloodstream
infections in COVID-19 patients in intensive
care: An alarming “collateral effect.” J
Glob Antimicrob Resist. 2020; 23:290–1.
- Amarsy R, Trystram D, Cambau E et al. Surging
bloodstream infections and antimicrobial
resistance during the first wave of COVID–19: a
study in a large multihospital institution in
the Paris region. Int J Infect Dis. 2022;
114:90–6.
- Giacobbe DR, Battaglini D, Ball L, Brunetti I,
Bruzzone B, Codda G, et al. Bloodstream
infections in critically ill patients with
COVID-19. Eur J Clin Invest. 2020;50(10):e13319.
- Polly M, De Almeida BL, Lennon RP, Cortês MF,
Costa SF, Guimarães T. Impact of the COVID-19
pandemic on the incidence of multidrug-resistant
bacterial infections in an acute care hospital
in Brazil. Am J Infect Control. 2022;50(1):32–8.
- Gajic I, Jovicevic M, Popadic V et al. The
emergence of multi-drug-resistant bacteria
causing healthcare-associated infections in
COVID-19 patients: a retrospective multi-centre
study. J Hosp Infect. 2023; 137:1–7.
- Budhiraja S, Tarai B, Jain D et al. Secondary
infections modify the overall course of
hospitalized patients with COVID-19: a
retrospective study from a network of hospitals
across North India. IJID Reg. 2022;
3:44–53.
- Chowdhary A, Tarai B, Singh A, Sharma A.
Multidrug-Resistant Candida auris Infections in
Critically Ill Coronavirus Disease Patients,
India, April–July 2020. Emerg Infect Dis. 2020;26(11):2694–6.
- Vijay S, Bansal N, Rao BK et al. Secondary
Infections in Hospitalized COVID-19 Patients:
Indian Experience. Infect Drug Resist. 2021;14:1893–903.
- Lim C, Hantrakun V, Teerawattanasook N et al.
Impact of low blood culture usage on rates of
antimicrobial resistance. J Infect.
2021;82(3):355–62.
|
Supplementary Table 1: Univariate
analysis for mortality among patients
with monomicrobial GNBSIs (n=375)
|
|
7-day mortality
|
30-day mortality
|
|
HR
|
P
|
95% CI
|
HR
|
P
|
95% CI
|
|
Male
|
1.073
|
0.694
|
0.756-1.523
|
1.034
|
0.837
|
0.750-1.426
|
|
Total length of hospital stay
|
0.875
|
0.000
|
0.845-0.906
|
0.895
|
0.000
|
0.869-0.921
|
|
Age
|
1.009
|
0.120
|
0.998-1.020
|
1.011
|
0.031
|
1.001-1.022
|
|
Fever
|
0.552
|
0.001
|
0.389-0.785
|
0.553
|
0.000
|
0.400-0.763
|
|
Diabetes mellitus
|
0.788
|
0.181
|
0.557-1.116
|
0.793
|
0.154
|
0.576-1.091
|
|
Hypertension
|
0.849
|
0.358
|
0.600-1.203
|
0.822
|
0.229
|
0.596-1.132
|
|
Ischemic heart disease
|
1.178
|
0.478
|
0.750-1.850
|
1.230
|
0.323
|
0.816-1.854
|
|
Chronic liver disease
|
1.944
|
0.006
|
1.205-3.136
|
1.813
|
0.012
|
1.141-2.880
|
|
Covid-19 infection
|
1.697
|
0.009
|
1.141-2.523
|
1.876
|
0.001
|
1.314-2.679
|
|
Chronic kidney disease
|
0.843
|
0.439
|
0.548-1.298
|
0.825
|
0.354
|
0.550-1.239
|
|
Obstructive airway disease
|
0.734
|
0.498
|
0.300-1.796
|
0.901
|
0.788
|
0.422-1.925
|
|
Immunosuppressive drugs
|
1.445
|
0.046
|
1.006-2.074
|
1.617
|
0.004
|
1.166-2.241
|
|
Malignancy
|
1.002
|
0.992
|
0.638-1.574
|
1.084
|
0.698
|
0.723-1.625
|
|
Source
|
|
0.006
|
|
|
0.004
|
|
|
Urinary tract
|
0.485
|
0.019
|
0.266-0.886
|
0.420
|
0.002
|
0.230-0.728
|
|
Lungs
|
1.889
|
0.012
|
1.152-3.099
|
1.679
|
0.038
|
1.029-2.594
|
|
Skin and soft tissue
|
0.595
|
0.468
|
0.147-2.418
|
0.669
|
0.464
|
0.206-2.053
|
|
Abdomen
|
1.153
|
0.808
|
0.365-3.643
|
0.865
|
0.769
|
0.267-2.655
|
|
Unknown
|
1 (Ref)
|
|
|
|
|
Microbiology
|
|
0.005
|
|
|
0.004
|
|
|
Escherichia coli
|
1.77
|
0.670
|
0.557-2.486
|
0.966
|
0.916
|
0.508-1.836
|
|
Klebsiella pneumoniae
|
1.756
|
0.128
|
0.850-3.626
|
1.320
|
0.381
|
0.709-2.457
|
|
Acinetobacter species
|
2.773
|
0.007
|
1.330-5.783
|
2.355
|
0.007
|
1.258-4.410
|
|
Pseudomonas aeruginosa
|
0.975
|
0.967
|
0.300-3.167
|
1.048
|
0.925
|
0.398-2.758
|
|
Other Enterobacterales
|
0.990
|
0.982
|
0.402-2.435
|
0.951
|
0.896
|
0.447-2.023
|
|
Other GNB
|
1.906
|
0.410
|
0.412-8.824
|
1.363
|
0.684
|
0.307-6.042
|
|
Other Non-fermenting GNB
|
1(Ref)
|
|
|
|
|
Carbapenem resistance
|
|
0.000
|
|
|
0.000
|
|
|
CRA-GNBSI
|
2.956
|
0.000
|
1.928-4.531
|
2.823
|
0.000
|
1.882-4.235
|
|
CRKP – GNBSI
|
1.466
|
0.134
|
0.889-2.419
|
1.526
|
0.067
|
0.970-2.400
|
|
Other CRE – GNBSI
|
0.917
|
0.956
|
0.415-2.206
|
1.291
|
0.449
|
0.667-2.499
|
|
Other CR-GNBSI
|
0.695
|
0.479
|
0.253-1.906
|
0.883
|
0.770
|
0.385-2.028
|
|
Carbapenem susceptible GNBSI
|
1 (Ref)
|
1 (Ref)
|
|
Ceftriaxone administration
|
0.758
|
0.246
|
0.475-1.211
|
0.998
|
0.991
|
0.672-1.481
|
|
Piperacillin tazobactam administration
|
0.946
|
0.754
|
0.668-1.340
|
0.990
|
0.949
|
0.719-1.362
|
|
Meropenem administration
|
1.746
|
0.003
|
1.206-2.527
|
1.753
|
0.002
|
1.239-2.479
|
|
Tigecycline administration
|
1.503
|
0.118
|
0.902-2.505
|
1.459
|
0.105
|
0.925-2.302
|
|
Polymyxin B administration
|
1.317
|
0.240
|
0.832-2.086
|
1.317
|
0.191
|
0.872-1.989
|
|
Central venous catheter insertion
|
0.711
|
0.086
|
0.481-1.050
|
0.876
|
0.444
|
0.624-1.230
|
|
Mechanical ventilation
|
2.833
|
0.000
|
1.997-4.018
|
3.412
|
0.000
|
2.467-4.719
|
|
Vasopressor administration
|
6.625
|
0.000
|
4.410-9.953
|
6.992
|
0.000
|
4.799-10.187
|
|
ICU admission
|
13.209
|
0.000
|
6.162-28.316
|
18.375
|
0.000
|
8.117-41.593
|
|
*P<0.1 was included in the
multivariate analysis
Abbreviations: GNBSI,
Gram negative bacterial blood stream
infection; GNB, Gram negative bacteria;
ICU, Intensive care unit; CRA, carbapenem
resistant Acinetobacter species;
CRKP, carbapenem resistant Klebsiella
pneumoniae; CRE, carbapenem
resistant Enterobacterales; CR-GNBSI,
carbapenem resistant Gram-negative
bacterial blood stream infection
|
|
Supplementary Table 2: Univariate
analysis for mortality among patients
with an event of ICU admission during
hospital stay (n=232)
|
|
7-day mortality
|
30-day mortality
|
|
HR
|
P
|
95% CI
|
HR
|
P
|
95% CI
|
|
Male
|
1.010
|
0.957
|
0.705-1.447
|
1.007
|
0.234
|
0.996-1.018
|
|
LOS
|
0.885
|
0.000
|
0.857-0.913
|
0.895
|
0.000
|
0.871-0.919
|
|
Age
|
1.003
|
0.560
|
0.992-1.015
|
0.973
|
0.872
|
0.701-1.351
|
|
Fever
|
0.748
|
0.117
|
0.520-1.076
|
0.714
|
0.046
|
0.514-0.994
|
|
Diabetes mellitus
|
0.770
|
0.153
|
0.538-1.102
|
0.756
|
0.092
|
0.546-1.047
|
|
Hypertension
|
0.737
|
0.095
|
0.515-1.055
|
0.703
|
0.035
|
0.507-0.975
|
|
Ischemic heart disease
|
0.851
|
0.511
|
0.527-1.376
|
0.901
|
0.637
|
0.586-1.387
|
|
Chronic liver disease
|
1.610
|
0.063
|
0.975-2.659
|
1.533
|
0.085
|
0.943-2.491
|
|
Covid-19 infection
|
1.146
|
0.506
|
0.767-1.711
|
1.259
|
0.208
|
0.879-1.804
|
|
Chronic kidney disease
|
0.969
|
0.889
|
0.624-1.506
|
0.944
|
0.786
|
0.624-1.428
|
|
Obstructive airway disease
|
0.662
|
0.366
|
0.240-1.620
|
0.799
|
0.562
|
0.374-1.708
|
|
Immunosuppressive drugs
|
1.060
|
0.759
|
0.731-1.537
|
1.210
|
0.261
|
0.868-1.687
|
|
Malignancy
|
1.098
|
0.702
|
0.679-1.775
|
1.226
|
0.346
|
0.802-1.875
|
|
Source
|
|
0.356
|
|
|
0.333
|
|
|
Urinary tract
|
0.667
|
0.225
|
0.347-1.284
|
0.566
|
0.072
|
0.304-1.053
|
|
Lungs
|
1.237
|
0.412
|
0.744-2.055
|
1.105
|
0.677
|
0.690-1.769
|
|
Skin and soft tissue
|
0.589
|
0.459
|
0.145-2.394
|
0.621
|
0.417
|
0.197-1.961
|
|
Abdomen
|
1.979
|
0.246
|
0.625-6.266
|
1.355
|
0.605
|
0.428-1.295
|
|
Unknown
|
1 (Ref)
|
1 (Ref)
|
|
Microbiology
|
|
0.106
|
|
|
0.106
|
|
|
Escherichia coli
|
1.210
|
0.619
|
0.571-2.565
|
0.987
|
0.969
|
0.518-1.883
|
|
Klebsiella pneumoniae
|
1.527
|
0.255
|
0.737-3.166
|
1.169
|
0.624
|
0.627-2.180
|
|
Acinetobacter species
|
2.073
|
0.053
|
0.989-4.345
|
1.793
|
0.070
|
0.954-3.370
|
|
Pseudomonas aeruginosa
|
0.599
|
0.442
|
0.162-2.212
|
0.683
|
0.469
|
0.243-1.917
|
|
Other Enterobacterales
|
0.974
|
0.955
|
0.386-2.453
|
0.937
|
0.868
|
0.434-2.022
|
|
Other GNB
|
2.247
|
0.301
|
0.484-10.435
|
1.840
|
0.423
|
0.413-8.198
|
|
Other Non-fermenting GNB
|
1 (ref)
|
|
|
|
|
Carbapenem resistance
|
|
0.008
|
|
|
0.027
|
|
|
CRA-GNBSI
|
1.875
|
0.005
|
1.204-2.918
|
1.766
|
0.008
|
1.164-2.681
|
|
CRKP – GNBSI
|
0.978
|
0.930
|
0.590-1.620
|
0.999
|
0.995
|
0.633-1.575
|
|
Other CRE – GNBSI
|
0.600
|
0.232
|
0.259-1.387
|
0.793
|
0.492
|
0.409-1.537
|
|
Other CR-GNBSI
|
0.499
|
0.178
|
0181-1.373
|
0.629
|
0.275
|
0.273-1.446
|
|
Carbapenem susceptible GNB
|
1 (Ref)
|
|
|
Ceftriaxone administration
|
0.865
|
0.547
|
0.540-1.386
|
1.063
|
0.763
|
0.714-1.583
|
|
Piperacillin tazobactam administration
|
0.912
|
0.615
|
0.638-1.305
|
0.935
|
0.685
|
0.675-1.295
|
|
Meropenem administration
|
1.083
|
0.681
|
0.739-1.587
|
1.093
|
0.626
|
0.765-1.561
|
|
Tigecycline administration
|
0.815
|
0.458
|
0.474-1.400
|
0.849
|
0.490
|
0.533-1.352
|
|
Polymyxin B administration
|
0.797
|
0.353
|
0.493-1.288
|
0.819
|
0.358
|
0.535-1.254
|
|
Central venous catheter insertion
|
0.474
|
0.000
|
0.318-0.706
|
0.561
|
0.001
|
0.396-0.794
|
|
Mechanical ventilation
|
1.250
|
0.226
|
0.871-1.794
|
1.480
|
0.021
|
1.061-2.067
|
|
Vasopressor administration
|
2.815
|
0.000
|
1.820-4.352
|
3.005
|
0.000
|
2.017-4.479
|
|
*P<0.1 was included in the
multivariate analysis
Abbreviations: GNBSI,
Gram negative bacterial blood stream
infection; GNB, Gram negative bacteria;
ICU, Intensive care unit; CRA, carbapenem
resistant Acinetobacter species;
CRKP, carbapenem resistant Klebsiella
pneumoniae; CRE, carbapenem
resistant Enterobacterales; CR-GNBSI,
carbapenem resistant Gram-negative
bacterial blood stream infection
|
|



















