|
Introduction
Primary
testicular follicular lymphoma (PTFL) is a rare
neoplasm of germinal centre B cells confined to
the testis. It occurs almost exclusively in
children and young adults and carries excellent
prognosis. It is a unique extranodal variant of
follicular lymphoma presenting as stage 1E
disease. They differ biologically from nodal FL in
that they lack evidence of the BCL2
translocation.[1] They are of high cytological
grade, usually grade 3A.[2,3] Microscopically, TFL
has a follicular or follicular and diffuse pattern
and is composed predominantly of centroblasts.
Although not recognized as a separate entity, the
4th edition of the World Health
Organization (WHO) Classification of Tumors of
Hematopoietic and Lymphoid Tissues lists TFL as a
distinctive variant of follicular lymphoma.[4]
Case Report
A 5-year-old
apparently healthy and active boy, presented with
a painless right scrotal swelling of 5 months
duration which gradually increased in size. There
was no history of fever, night sweats, malaise,
weight loss or recent change in bowel habits. He
had no significant past medical or surgical
history. Antenatal and postnatal periods were
uneventful and he had a recorded birth weight of
2.9kg. He was immunized according to National
immunization schedule. There was no history of
malignancy in the family. On physical examination,
a 4x3cm non tender swelling was felt confined to
the right testis which was soft to firm in
consistency. Cord structures were normal. Inguinal
nodes were not palpable. Examination of the left
testis and of other systems was unremarkable.
Ultrasound of
scrotum showed an enlarged hypoechoic right testis
with increased vascularity. No obvious focal
lesions were found. Routine hematological
examination was unremarkable. Tumors markers –
Serum Alpha Feto Protein (AFP), Human Chorionic
Gonadotropin (HCG) and Lactate Dehydrogenase (LDH)
were within normal limits. Chest X-ray was
unremarkable. MRI of the scrotum revealed an
enlarged right testis measuring 28x14mm, and was
suggestive of right epididymo-orchitis.
The child underwent
right high inguinal orchidectomy by Chevassu
procedure. Intra-operatively, right testis was
bulky measuring 4x3cm, with normal cord
structures. Frozen section report was given as
malignant small round blue cell tumor.
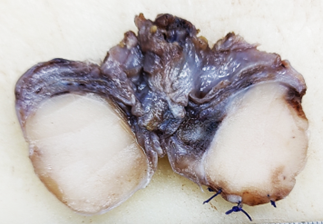
On cut section, the
testis was replaced by a grey-white tumor
measuring 2.5 x 2 x 1.5 cm and was limited to
within the testis parenchyma. The epididymis and
spermatic cord appeared grossly free of the tumor.
The tunica vaginalis and tunica albuginea were
grossly uninvolved (Fig.1).
|
| Figure
1: Cut section, testis shows an irregular
grey white lesion measuring 2.5x2x1.5cm,
limited to testis. |
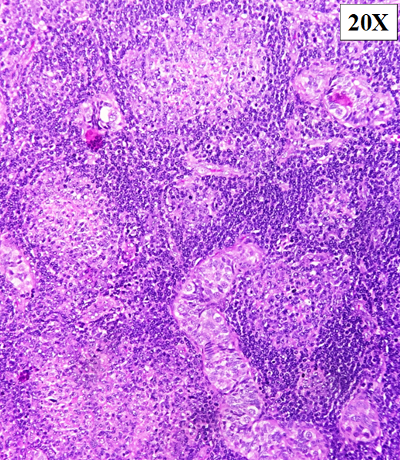
On microscopy,
sections from the tumor showed atypical lymphoid
proliferation seen predominantly in a
nodular/follicular pattern. The atypical lymphoid
cells showed variable morphology and were composed
of small to medium cleaved cells and admixed large
centroblasts cells (>15/hpf). The follicles
were seen back-to-back and seen permeating in
between and inside the seminiferous tubules (Fig.
2-3). Scattered mitoses were seen within the
tumor. No necrosis was identified.
|
|
| Figure
2: Haematoxylin & eosin staining:
Atypical lymphoid proliferation seen
predominantly in a nodular/follicular
pattern (10X & 40X). |
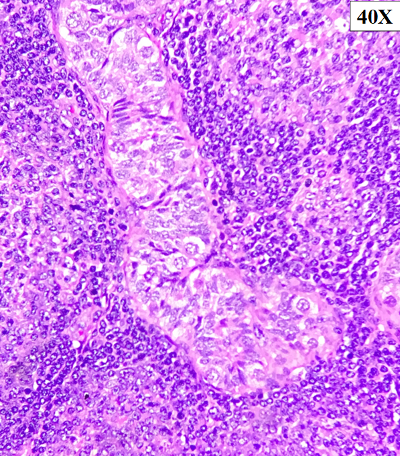
Figure
3: The follicles are seen back-to-back and
seen permeating in-between and inside the
seminiferous tubules These atypical
lymphoid cells show variable morphology
and are composed of small to medium
cleaved cell and admixed large
centroblasts-like cells (>15/hpf),40x
|
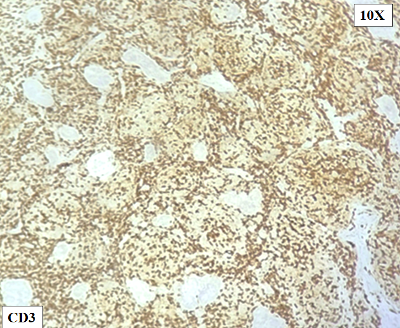
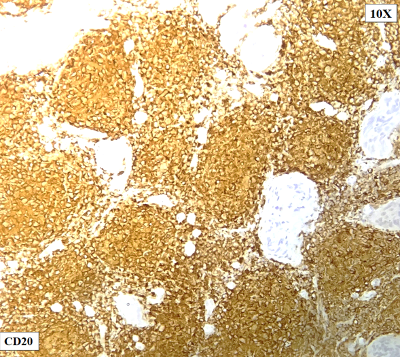
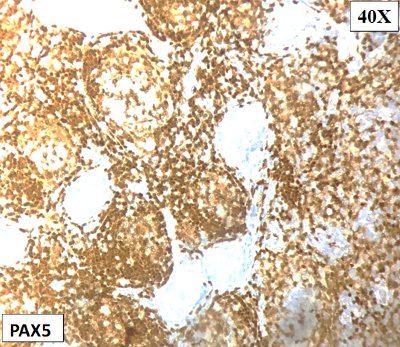
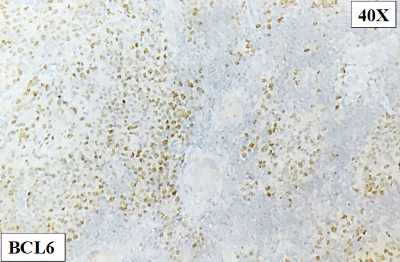
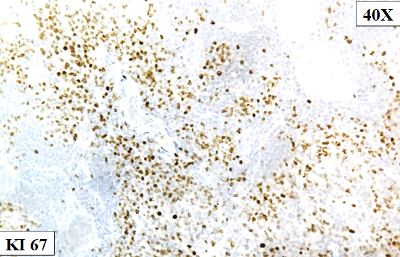
On
immunohistochemistry, the atypical lymphoid cell
follicles / nodules were diffuse positive for
CD20, PAX5 and BCL6. They were negative for CD10,
CD117, CD30, CD34, MPO, Tdt, BCL2 and MUM1(Fig.
4-8). The Ki 67/MIB1 labeling index was
approximately 50-60% in the highest proliferating
regions (Fig. 9).
|
|
| Figure
4: CD3 stains the T cells in the periphery
of the follicles. |
Figure
5: The atypical lymphoid cell follicles /
nodules are diffuse positive for CD20,
CD79a(Not shown) |

|
|
| Figure
6: Negative for BCL2(), CD117, CD30, CD34,
MPO, Tdt, and MUM1(Not shown). |
Figure
7: Positive for PAX5 |
|
|
| Figure 8: Positive for
BCL6 |
Figure
9: Ki- 67 indicates a moderate to high
proliferation rate (50-60%) |
The final impression
was given as a B-cell non-Hodgkin lymphoma with a
follicular architecture and a diagnosis of Primary
Testicular Follicular Lymphoma was considered.
|
Table 1: Clinical Immunophenotype
and translocation among reported cases
of Follicular Non-Hodgkin Lymphoma of
the Testes
|
|
Series
|
Site
|
Age
|
CD3
|
CD20
|
CD5
|
CD10
|
CD23
|
BCL6
|
MIB-1
|
BCL-2
|
t(14;18) (q32;q21)/IGH-BCL2
|
|
Kevin et al(7)
|
Testis
|
3y
|
-
|
+
|
NA
|
+
|
NA
|
+
|
Moderately high
|
-
|
Not applicable
|
|
Pileri et al(10)
|
Testis
|
4y
|
-
|
+
|
NA
|
+
|
NA
|
+
|
70%
|
-
|
-
|
|
Lu et al(11)
|
Testis
|
6y
|
-
|
+
|
NA
|
+
|
NA
|
+
|
NA
|
-
|
-
|
|
Bacon et al(6)
|
Testis
|
5y
|
-
|
+
|
NA
|
+
|
NA
|
+
|
NA
|
-
|
-
|
|
Lones(3)
|
Testis
|
3 to 11y
|
NA
|
+
|
NA
|
+
|
NA
|
+
|
NA
|
-
|
-
|
|
Finn(8)
|
Testis
|
3 to 10
|
_
|
+
|
_
|
+
|
_
|
+
|
NA
|
-
|
-
|
|
Garces(1)
|
Testes
|
NA
|
-
|
+
|
-
|
+
|
+
|
+
|
40-70%
|
-
|
-
|
|
Vincenzo Tralongo(12)
|
epididymis
|
90y
|
-
|
+
|
NA
|
+
|
-
|
+
|
60%
|
-
|
present
|
|
Present study
|
Testes
|
5y
|
-
|
+
|
-
|
+
|
-
|
-
|
50-60%
|
-
|
NA
|
|
Table 2: Differences between
nodal and testicular follicular lymphoma
|
|
Nodal follicular lymphoma
|
Testicular follicular lymphoma
|
|
Age (median)
|
Adults and elder (sixth decade)
|
Children and young adults
|
|
Affected sites
|
Lymph nodes with extranodal spread
|
Testicle and adnexa
|
|
Symptoms
|
Generalized lymphadenopathy
|
Painless mass
|
|
Gross appearance
|
Discrete mass or complete effacement
|
Discrete mass or diffuse involvement
|
|
Histologic grade
|
Grades 1 - 3
|
Grade 3
|
|
CD10
|
Variable
|
Variable
|
|
BCL2
|
Usually, positive
|
Negative
|
|
IGH-BCL2 rearrangements
|
Present, up to 90%
|
Negative
|
Discussion
This study presents
a case of primary testicular follicular lymphoma
in a 5-year-old child. The lymphoma exhibited
characteristic morphologic features of follicular
lymphoma and expressed the germinal center marker
BCL6. However, the neoplastic cells did not
express BCL2. Distinction from a reactive process
was possible owing to the unique morphology of the
lesion and the presence of an extensive, dense
infiltrate of BCL-6 positive atypical B
lymphocytes. A diagnosis of primary testicular
lymphoma was considered as the disease was
primarily present in the testis and a concomitant
clinical and radiological evaluation did not
reveal any other site of involvement. In addition,
the lymphoma is morphologically,
immunohistochemically, similar to the other cases
described, and secondary involvement of the testis
by follicular lymphoma is exceptionally rare.[5,6]
Primary testicular
lymphoma is uncommon, constituting approximately
1% of all lymphomas and 2% to 5% of all testicular
tumors.[6] Typically affecting adult men, it is
rarely seen in children.[7,8,9] About 80% to 98%
of cases are Primary testicular diffuse large B
cell lymphoma (PT-DLBCL).[1] Secondary testicular
involvement is seen commonly in blastic lymphomas.
The true incidence of PTFL is unknown owing to its
rarity. Nevertheless, about 25 cases have been
reported in the literature so far.[1]
Clinically, it
manifests as unilateral painless testicular
enlargement. Ultrasound appearance is diffuse or
well-delineated hypoechoic unilateral mass lesion
with increased vascularity. Comprehensive staging
investigations almost invariably show extra nodal
and organ-confined disease.
Grossly they are
tan/white fleshy ill-defined tumors primarily
located in the testicular parenchyma with frequent
extension to the epididymis. Microscopically, the
distorted testicular parenchyma shows dense
lymphoid infiltrate with a follicular growth
pattern and are predominantly composed of
centroblasts and fewer mixed centrocytes. The
neoplastic follicles permeate among seminiferous
tubules. Immunohistochemically, tumor cells are
positive for B-cell lineage markers – CD19, CD20
and CD79a. They express at least one of the two
germinal center-associated antigens CD10 and BCL6.
BCL2 is characteristically negative in the
neoplastic cells. The proliferation index assessed
by Ki-67 is moderately high ranging from 40% to
80%.
Genetically, TFL
characteristically lacks the t(14;18)/IGH-BCL2.
Mutations commonly involve EZH2, EGFR, IRF8,
PABPC1, KMT2D, TNFRSF14.[1] FLs lacking BCL2
rearrangement display CNAs and mutations similar
to BCL2-R FL, but with different frequencies.
However, in contrast to BCL2-R FL which expresses
GC B-cell signatures, the gene expression profile
of FLs lacking BCL2 rearrangement is reminiscent
of late/post-GC cells.
Differentiating PTFL
from reactive follicular hyperplasia in the course
of chronic orchitis poses as a diagnostic
challenge. Because the lack of BCL2 protein
expression and BCL2 gene rearrangement can make
the diagnosis of FL less obvious, a series of
parameters should always be considered. This
includes the absence of previous infectious
diseases, lack of inflammation or granulomas in
the epididymis and spared testis, back-to-back
follicular growth pattern, non-polarized germinal
centers, Ig light chain restriction, and the
detection of a monoclonal Ig gene rearrangement.
Microscopically neoplastic follicles
characteristically permeate in between and within
the seminiferous tubules. These follicles lack
well-defined mantle cuffs and germinal centre
polarisation.
A comprehensive
review of the literature published found cases of
testicular tumors of follicular Non-Hodgkin
Lymphoma (NHL) in 10 children. As seen in all
prior cases reported, our patient was negative for
BCL-2. Table 1 outlines how our studies compare
with the prior cases of primary testicular
follicular NHL. Each child was reported to have
only localized follicular NHL of a testis. Table 2
depicts differences between nodal follicular
lymphoma and testicular follicular lymphoma
Treatment of PTFL
involves a combined approach of unilateral
orchidectomy and anthracycline-containing
chemotherapy. An excellent prognosis is usually
achieved without recurrence after long-term follow
up.
References
- Garces S, Xu J, Li S. Primary testicular
follicular lymphoma. Human Pathology
Reports. 2022 Mar 1;27:300606.
- Liu Q, Salaverria I, Pittaluga S, Jegalian AG,
Xi L, Siebert R, Raffeld M, Hewitt SM, Jaffe ES.
Follicular lymphomas in children and young
adults: a comparison of the pediatric variant
with usual follicular lymphoma. The American
Journal of Surgical Pathology. 2013
Mar;37(3):333.
- Lones MA, Raphael M, McCarthy K, Wotherspoon
A, Terrier-Lacombe MJ, Ramsay AD, MacLennan K,
Cairo MS, Gerrard M, Michon J, Patte C. Primary
follicular lymphoma of the testis in children
and adolescents. Journal of Pediatric
Hematology/Oncology. 2012 Jan;34(1):68.
- Swerdlow SH. World Health Organization
classification of tumours of the haematopoietic
and lymphoid tissues. In Swerdlow SH, Campo E,
Harris NL et al. (Eds.) Postgraduate
Haematology. 4th ed. 2017. pp986-8.
- Case Jr DC, Waldbaum R, Vinciguerra V, Tomao
F. Malignant lymphoma with genitourinary
symptoms. Urology. 1975 May
1;5(5):654-7.
- Bacon CM, Ye H, Diss TC, McNamara C, Kueck B,
Hasserjian RP, Rohatiner AZ, Ferry J, Du MQ,
Dogan A. Primary follicular lymphoma of the
testis and epididymis in adults. The
American Journal of Surgical Pathology. 2007
Jul 1;31(7):1050-8.
- Heller KN, Teruya-Feldstein J, La Quaglia MP,
Wexler LH. Primary follicular lymphoma of the
testis: excellent outcome following surgical
resection without adjuvant chemotherapy. J
Pediatr Hematol Oncol. 2004;26(2):104-107.
- Finn LS, Viswanatha DS, Belasco JB et al.
Primary follicular lymphoma of the testis in
childhood. Cancer. 1999 Apr
1;85(7):1626-35.
- Kay R. Prepubertal testicular tumor registry.
Urologic Clinics of North America. 1993
Feb 1;20(1):1-5.
- Pileri SA, Sabattini E, Rosito P et al.
Primary follicular lymphoma of the testis in
childhood: an entity with peculiar clinical and
molecular characteristics. Journal of
Clinical Pathology. 2002 Sep
1;55(9):684-8.
- Lu DI, Medeiros LJ, Eskenazi AE, Abruzzo LV.
Primary follicular large cell lymphoma of the
testis in a child. Archives of Pathology and
Laboratory Medicine. 2001 Apr
1;125(4):551-4.
- Tralongo V, Becchina G, Nagar C et al. Primary
follicular lymphoma of the epididymis positive
for t (14; 18)(q32; q21)/IGH-BCL2 and negative
for BCL2 protein expression: a case report. Journal
of Medical Case Reports. 2012 Dec;6:1-5.
|



















